In the relentless quest for weight loss, society has often overlooked the most vital aspects of true health and well-being. The narrative around weight loss has long been dominated by simplistic equations—calories in versus calories out—and narrow ideals that prize thinness above true metabolic integrity. As a result, we have reduced our health aspirations to chasing ever-smaller numbers on a scale, believing that a shrinking body automatically equates to a healthier one. Scales become the ultimate judges of success, calories are demonized, and bodies are measured solely in terms of appearance rather than function, resilience, or vitality.
But beneath this cultural obsession lies a deeper and more revolutionary truth—one that challenges the conventional paradigm. Real, lasting, and meaningful weight management is not about deprivation; it’s about preservation. It’s about holding onto what matters most—muscle, bone, mitochondrial health, hormonal harmony, and cognitive clarity—while shedding excess fat and inflammation. It’s about cultivating a system that’s robust and vibrant, not simply lighter. This shift in mindset reframes weight loss as a process of gaining strength, longevity, and inner stability—not just losing weight for aesthetics or short-term gratification.
Far too many people have embarked on weight loss journeys only to end up depleted—physically, emotionally, and metabolically. We must ask ourselves: are we pursuing health, or simply thinness? Sustainable weight management is about optimizing every system in the body, not starving them. The real prize isn’t dropping sizes—it’s gaining strength, resilience, and an enduring quality of life.
Lean Mass: The Metabolic Gold
Muscle is not a luxury; it’s a necessity. In the evolving conversation around weight loss, preserving lean body mass—particularly skeletal muscle—has emerged as a critical yet often neglected priority. Beyond aesthetics or performance, lean mass is fundamentally tied to overall metabolic health, insulin sensitivity, immune resilience, and even mental vitality. Muscle tissue is a metabolically active organ that not only consumes energy at rest but also acts as a powerful regulator of systemic glucose levels, inflammation, and hormonal balance. As we age, the gradual loss of muscle mass—known as sarcopenia—can quietly shift the balance from healthy weight loss to a cascade of health challenges including insulin resistance, frailty, increased fall risk, and accelerated biological aging [1].
When people pursue aggressive calorie restriction or over-exercise without strategic support, the body often breaks down muscle to meet its energy demands. This muscle loss weakens not just physical strength but the very foundation of metabolic health. Lean muscle functions as a reservoir for glucose and amino acids, providing both energy and essential substrates during times of stress or caloric deficit [2]. Just as importantly, muscle acts as an endocrine organ, releasing beneficial signaling molecules called myokines during contraction. These myokines exert anti-inflammatory, anti-diabetic, and even anti-tumor effects, helping the body maintain homeostasis across systems—from the brain to the liver and immune system [3]. Thus, muscle isn’t just something to “tone up”—it’s an essential, active player in the body’s weight regulation machinery.
To protect muscle during weight loss, several interventions must work in tandem. First and foremost is resistance training, ideally performed at least three to four times per week. Compound movements like squats, deadlifts, and push-pull sequences activate large muscle groups and stimulate protein synthesis, which not only prevents loss but may build new muscle even in a calorie deficit. Second, adequate protein intake is crucial—aiming for 1.2 to 2.0 grams of protein per kilogram of body weight per day is a clinically supported benchmark [4]. This becomes especially important with age, when anabolic resistance increases the protein requirements for maintaining muscle tissue. Supplementation with branched-chain amino acids (BCAAs), particularly leucine, can help preserve lean mass during weight loss and intermittent fasting, providing targeted stimulation of mTOR for muscle synthesis without excessive overall calories [5].
Beyond protein and training, strategic support with nutrients like magnesium citrate can improve muscular recovery and reduce post-exercise inflammation.
Similarly, riboflavin (B2) and niacin (B3), taken in higher therapeutic doses, can enhance mitochondrial efficiency within muscle cells—optimizing ATP production, reducing oxidative stress, and boosting endurance capacity. For individuals pursuing advanced mitochondrial support, compounds like methylene blue are emerging as adjuncts that enhance electron transport and reduce cellular fatigue, potentially preserving muscle function during caloric restriction.
Resveratrol, a polyphenol found in red grapes and berries, also supports lean mass by activating the SIRT1-AMPK pathway, which indirectly inhibits mTOR overactivation while enhancing mitochondrial biogenesis and insulin sensitivity. Its mild mTOR modulation may allow for fat loss while preserving muscle, especially in those undergoing intermittent fasting or ketogenic nutrition. Moreover, resveratrol’s anti-inflammatory and antioxidant properties help blunt catabolic signals that can otherwise accelerate muscle breakdown in calorie-deficit states.
Another crucial yet underappreciated tool in maintaining lean mass during weight management is pregnenolone, the master neurosteroid and precursor to hormones like DHEA, progesterone, testosterone, and cortisol. During extended stress, fasting, or caloric restriction, the body may shunt hormone production toward cortisol at the expense of anabolic and neuroprotective pathways—a process known as pregnenolone steal. Supplementing with low physiological doses of pregnenolone may help buffer against this depletion, promoting more balanced endocrine signaling. This can support muscle protein synthesis indirectly by stabilizing testosterone and DHEA levels, while also improving cognitive clarity and motivation—factors often compromised during prolonged dieting. Pregnenolone’s ability to modulate GABA and NMDA receptors also contributes to better sleep and recovery, both of which are essential for muscle preservation and repair.
Bone Density: The Silent Strength
Bone may seem inert and structural, but in truth, it is a dynamic, living tissue that communicates constantly with the rest of the body. In the context of weight loss, bone health is often an afterthought—but it shouldn’t be. Weight loss that leads to the erosion of bone mass can quietly accelerate biological aging, especially in vulnerable populations such as postmenopausal women, individuals on restrictive diets, and the elderly [6]. The consequences aren’t merely cosmetic or mobility-related; reduced bone density increases the risk of fractures, chronic pain, and long-term disability. Moreover, it often happens silently, without overt symptoms, until a major injury reveals the underlying fragility.
Emerging science shows that the relationship between bone and metabolism is far more intimate than previously understood. Bone is not just scaffolding—it’s a metabolically active endocrine organ. Hormones like osteocalcin, secreted by bone-forming osteoblasts, have systemic effects, including enhancing insulin sensitivity, increasing testosterone production, and improving mitochondrial function [7]. This means that bone health is intertwined with the very processes we seek to optimize during weight loss: hormonal balance, energy production, and metabolic control. Compromising bone in the pursuit of fat loss may paradoxically impair the body’s ability to maintain that loss.
To preserve and build bone during weight loss, several strategies should be prioritized. Weight-bearing exercises—especially resistance training, yoga, hiking, and bodyweight movements—stimulate the mechanical loading needed to trigger bone remodeling. Unlike cardio alone, resistance exercises apply specific stress to bones, prompting adaptive strengthening and improved density over time. This is essential not only for maintaining skeletal strength but also for enhancing joint integrity and posture.
Nutritional support is equally vital. Vitamin D3 enhances calcium absorption, while vitamin K2 helps shuttle calcium into bones and away from arteries—an often-overlooked nuance in preventing vascular calcification. Among minerals, magnesium citrate stands out due to its superior bioavailability and dual action: it supports both bone mineralization and muscle relaxation, making it invaluable during weight loss regimens that include increased physical training [8]. Magnesium is also involved in over 300 enzymatic processes, many of which influence bone turnover, hormonal balance, and inflammation regulation.
Tracking progress through periodic DEXA scans is a smart and often underutilized strategy [9]. DEXA not only provides a snapshot of bone density but also differentiates between fat mass and lean mass, offering a more complete picture of body composition. For those serious about sustainable, health-promoting weight loss, knowing what’s being lost—fat, muscle, or bone—can be the difference between transformation and regression. Prioritizing bone health isn’t just about preventing fractures; it’s about safeguarding the silent strength that upholds metabolic vitality, mobility, and longevity.
The Role of Inflammation
Fat is not merely passive storage for excess calories—it is a hormonally active, immunologically significant organ system that communicates continuously with the rest of the body. In particular, visceral fat, the type that surrounds internal organs, acts as an endocrine organ that secretes a range of pro-inflammatory cytokines, including TNF-α (tumor necrosis factor alpha) and IL-6 (interleukin-6). These inflammatory mediators interfere with insulin receptor signaling, promoting systemic insulin resistance, dysregulated glucose metabolism, and chronic low-grade inflammation—hallmarks of metabolic syndrome and type 2 diabetes [10].
This persistent inflammatory milieu does more than just stall weight loss efforts; it actively degrades health on multiple fronts. Chronic inflammation damages the endothelial lining of blood vessels, accelerating the development of atherosclerosis and increasing cardiovascular risk. It also contributes to neuroinflammation, impairing cognitive performance, mood, and increasing the risk of neurodegenerative diseases such as Alzheimer’s. On a cellular level, inflammation directly impairs mitochondrial function, decreasing energy production, increasing reactive oxygen species (ROS), and promoting fatigue, aging, and metabolic inefficiency. Thus, inflammation is not just a symptom but a central driver of disease and weight regulation failure.
The solution lies in comprehensive inflammation control—not through suppressing the immune system, but by retraining and nourishing it through strategic interventions. A whole-food, anti-inflammatory diet is foundational. This includes generous intake of polyphenol-rich fruits and vegetables, fiber to feed beneficial gut bacteria, and omega-3 fatty acids from sources like walnuts, and fatty fish [11]. These nutrients help reduce the production of pro-inflammatory mediators and increase anti-inflammatory counterparts like IL-10 and adiponectin.
Beyond nutrition, lifestyle strategies such as mindful stress reduction, high-quality sleep, and intermittent fasting help rebalance the hypothalamic-pituitary-adrenal (HPA) axis and reduce cortisol-mediated inflammation [12]. Even moderate exercise can lower C-reactive protein (CRP) levels and stimulate anti-inflammatory myokines from skeletal muscle. These habits, when practiced consistently, form the foundation of an anti-inflammatory lifestyle.
Targeted supplementation provides another layer of support. Curcumin, the active compound in turmeric, inhibits the NF‑κB pathway, a master regulator of inflammation. Ginger, with its natural COX-2-inhibiting properties, also offers synergistic anti-inflammatory action. Of special interest is resveratrol, a plant-derived polyphenol that activates sirtuins, particularly SIRT1, which play a critical role in modulating inflammatory responses and mitochondrial function [13]. Resveratrol not only blunts the cytokine storm associated with visceral fat but also helps recalibrate the body’s inflammatory thermostat at a molecular level. Its dual role in mitochondrial protection and inflammatory modulation makes it an ideal candidate for individuals looking to reduce fat mass without compromising metabolic resilience.
By recognizing and targeting inflammation as a root cause—not just a side effect—of weight challenges, we lay the groundwork for more sustainable and meaningful health outcomes. Inflammation is not just a fire to be put out; it is a biological signal that, when properly interpreted and addressed, can guide us toward deeper healing, improved vitality, and longer-lasting weight regulation.
Liver Health: The Metabolic Command Center
Often overlooked in weight loss conversations, the liver plays a truly central role in regulating metabolism, hormone balance, detoxification, and fat processing. It is the body’s metabolic command center, coordinating an astonishing number of chemical reactions every day—more than 500 in total. From metabolizing carbohydrates and breaking down fats to neutralizing toxins and converting hormones, the liver is integral to maintaining internal balance. When the liver is sluggish or overburdened—due to poor diet, excessive alcohol, prescription drugs, or environmental toxins—its ability to process fats and regulate glucose declines. This dysfunction can create a metabolic bottleneck, making weight loss difficult, even in people who are otherwise eating well and exercising.
A compromised liver may allow fatty deposits to accumulate within its own tissue, a condition known as non-alcoholic fatty liver disease (NAFLD). NAFLD is now one of the most common liver conditions worldwide and is strongly associated with insulin resistance, metabolic syndrome, and central obesity [14]. As the liver struggles to detoxify and process nutrients efficiently, inflammation and oxidative stress can build up systemically, impairing not just fat loss but also energy levels, immune function, and hormonal health. Since the liver also helps metabolize estrogens and other steroid hormones, a sluggish liver can worsen estrogen dominance or hormonal imbalance, especially during perimenopause and andropause.
Supporting liver function is thus not just helpful—it is essential for healthy, sustainable weight management. A multipronged approach is best. Certain nutraceuticals are well-researched for their liver-protective properties. N-acetyl cysteine (NAC) boosts intracellular glutathione, the body’s master antioxidant, helping neutralize free radicals and support Phase II detoxification. Milk thistle (silymarin) has been shown to stabilize liver cell membranes and stimulate protein synthesis in hepatocytes, while alpha-lipoic acid supports both liver regeneration and insulin sensitivity [14]. These compounds create a protective biochemical environment that enhances fat metabolism and reduces oxidative damage.
Diet also plays a major role in liver health. Cruciferous vegetables—like broccoli, cauliflower, Brussels sprouts, and kale—are rich in glucosinolates, which stimulate Phase I and Phase II detox enzymes in the liver. These enzymes help convert lipophilic toxins into water-soluble forms that can be safely excreted [15]. The fiber in these vegetables also supports gut-liver axis function by binding toxins and facilitating regular elimination. Combined with sufficient hydration and deep, restorative sleep, these practices help ensure that the liver can efficiently clear metabolic byproducts and prevent the recirculation of toxins in bile.
In more complex or toxin-burdened individuals, intravenous (IV) detoxification strategies may be warranted. Chelation therapy, using agents like EDTA or N acetyl cycteine, under medical supervision, can assist in binding and removing heavy metals such as lead, mercury, and cadmium—substances known to impair mitochondrial function and enzyme activity in the liver. These toxins, when left unchecked, contribute not only to fatty liver and oxidative stress but also to hormonal disruption and sluggish metabolism. Regular assessments and guided detox protocols can significantly reduce this toxic load, often resulting in better energy, improved body composition, and enhanced clarity of mind.
By treating the liver as the metabolic powerhouse that it is, and not merely a silent filter, we create the internal conditions for real transformation. A well-functioning liver clears the path for hormone balance, inflammation reduction, effective fat burning, and ultimately, sustainable weight loss from the inside out.
Brain and Joint Health: Weight Loss Without the Cost
In the pursuit of rapid weight loss, it’s easy to focus exclusively on calories, exercise, and appearance—yet often forgotten are the two pillars of long-term vitality and quality of life: the brain and the joints. Unfortunately, overly aggressive caloric restriction or nutrient-poor diets can have unintended consequences that affect these critical systems. When the body is deprived of key macronutrients and micronutrients, it may cannibalize structural proteins, reduce neurotransmitter synthesis, and accelerate cartilage breakdown. The result? Cognitive fog, mood instability, and increased joint pain—symptoms that are not only discouraging but can derail even the most well-intentioned weight management plans.
The brain is metabolically demanding, consuming over 20% of the body’s energy at rest. It depends on a continuous supply of healthy fats, particularly long-chain omega-3 fatty acids (EPA and DHA), stable glucose levels, and micronutrients like B vitamins, magnesium, and antioxidants to maintain its functions—memory, mood regulation, focus, and neuroplasticity. Erratic blood sugar levels, inflammation, and oxidative stress can impair synaptic efficiency and neurotransmitter production, contributing to fatigue, depression, and anxiety. Similarly, joints require structural proteins such as collagen, as well as synovial fluid support from compounds like hyaluronic acid and methylsulfonylmethane (MSM) to stay resilient under mechanical load, especially during weight-bearing exercises.
To support both neurological and musculoskeletal systems during weight loss, a foundational approach includes ensuring sufficient intake of omega-3 fatty acids, particularly EPA and DHA, which are vital for neuronal membrane fluidity and the regulation of inflammation in both the brain and joints [16]. These fatty acids not only protect against neurodegeneration but have been shown in studies to improve mood, reduce anxiety, and support executive function. For those with dietary restrictions, high-quality fish oil or algae-based omega-3 supplements can provide these benefits with minimal caloric burden.
Incorporating low-impact, joint-friendly movement—such as swimming, yoga, Pilates, or brisk walking—provides the dual benefit of stimulating circulation and maintaining mobility without excessive mechanical strain. This movement also supports lymphatic drainage, which is essential for clearing inflammatory metabolites that can accumulate with tissue breakdown or detoxification.
One underappreciated but powerful ally in preserving both cognitive clarity and joint integrity is pregnenolone, a neurosteroid produced in the brain and adrenal glands. As the precursor to key hormones like DHEA, progesterone, testosterone, and cortisol, pregnenolone plays a central role in neuroendocrine balance. It modulates receptors such as GABA and NMDA, contributing to enhanced mood stability, reduced anxiety, and sharper mental performance. Additionally, some emerging research suggests that pregnenolone may influence connective tissue metabolism and joint resilience, potentially improving recovery and reducing pain perception during periods of weight loss and increased physical demand. By supporting neurohormonal homeostasis, pregnenolone can be a valuable tool for avoiding burnout and breakdown while pursuing body recomposition.
When weight loss is approached through a lens that honors the interconnectedness of brain and joint health, the result is a more sustainable, energizing, and empowering journey. Rather than sacrificing cognitive vitality and physical freedom, we can build a path to health that enhances both—allowing us to think, move, and feel better at every stage of transformation.
Mitochondria: Engines of Life
At the cellular level, weight management is mitochondrial management. These tiny, double-membraned organelles serve as the energy factories of our cells, converting nutrients into usable energy in the form of ATP through oxidative phosphorylation. Their efficiency—or lack thereof—directly dictates how well we burn fat, respond to stress, regulate inflammation, and maintain physical stamina. Impaired mitochondrial function doesn’t just lead to fatigue—it lies at the heart of insulin resistance, obesity, neurodegeneration, and accelerated aging [18]. In essence, when mitochondria fail, the whole body slows down—metabolically, cognitively, and immunologically.
Mitochondria do far more than produce energy; they also regulate apoptosis (programmed cell death), modulate the production of reactive oxygen species (ROS), and participate in the cellular stress response. In individuals with metabolic dysfunction, these mitochondria may become swollen, leaky, and inefficient—burning fuel poorly while spilling oxidative byproducts into the surrounding tissue. This “leaky battery” state can lead to a vicious cycle of inflammation, hormonal disruption, and impaired detoxification, all of which sabotage fat loss and degrade overall vitality. A sluggish mitochondrion doesn’t just stall your metabolism; it creates a state of biological inertia.
Optimizing mitochondrial function is therefore a cornerstone of sustainable weight loss, especially for individuals suffering from fatigue, brain fog, or metabolic resistance. One of the most exciting and non-invasive methods for enhancing mitochondrial health is red and near-infrared light therapy. By stimulating cytochrome c oxidase in the mitochondrial electron transport chain, photobiomodulation boosts ATP output, reduces inflammation, and can even trigger mitochondrial biogenesis—the creation of new mitochondria [19]. This makes red light therapy particularly valuable for those with sedentary lifestyles, injuries, or chronic pain who may not yet tolerate vigorous exercise.
Nutritional and nutraceutical interventions also play a powerful role in mitochondrial revitalization. Key compounds such as Coenzyme Q10 (CoQ10), PQQ, and L-carnitine work synergistically to enhance mitochondrial energy output, protect against oxidative damage, and shuttle fatty acids into mitochondria for oxidation. Among these, methylene blue is gaining interest as a novel “electron cycler”—acting as a bypass for damaged segments of the electron transport chain. By accepting and donating electrons, methylene blue improves redox cycling, enhances ATP production, and reduces ROS generation in compromised cells [20]. Used in microdoses, it has shown promise in conditions ranging from mitochondrial myopathy to cognitive decline, and could serve as a valuable tool in resistant weight loss and energy optimization strategies.
High-dose riboflavin (vitamin B2) and niacin (vitamin B3) offer additional mitochondrial support by functioning as precursors to FAD+ and NAD+, respectively—critical cofactors in enzymatic reactions that drive energy metabolism. These nutrients can be particularly helpful in individuals with mitochondrial fatigue, chronic illness, or those exposed to high levels of environmental toxins. Enhancing the NAD+/NADH ratio, a key marker of mitochondrial health, also promotes autophagy and DNA repair, further linking these vitamins to longevity pathways.
An often-underestimated ally in mitochondrial wellness is ascorbic acid (vitamin C). While best known as an antioxidant, vitamin C also plays a cofactor role in carnitine synthesis, collagen production (crucial for mitochondrial membrane integrity), and several steps within the TCA cycle, the energy-producing hub of the mitochondrion. Its antioxidant properties help buffer against mitochondrial-generated ROS, while also supporting adrenal function during periods of metabolic stress.
Lifestyle strategies round out the mitochondrial toolbox. Cold exposure, intermittent fasting, and high-intensity exercise all stimulate mitochondrial biogenesis and autophagy, creating a cellular environment optimized for fat burning and longevity [21]. Fasting in particular activates AMPK and PGC-1α, key regulators of energy balance and mitochondrial renewal, while simultaneously reducing insulin and inflammation.
When mitochondria thrive, the body gains the capacity to lose fat without losing energy, to detoxify without exhaustion, and to age without decline. By nourishing these intracellular engines, we ignite a foundation not only for sustained weight management, but also for vibrant, whole-body health.
Hormones: Conductors of Weight and Wellness
Hormones are the body’s master messengers, orchestrating nearly every physiological process, from metabolism and mood to sleep, libido, and fat storage. In the context of weight management, hormonal balance is not optional—it is foundational. Hormones act like conductors of a symphony: when in harmony, the body hums along with vitality and metabolic clarity; when imbalanced, even the best efforts in nutrition and exercise may falter. Key players like thyroid hormones, cortisol, insulin, estrogen, and testosterone form a dynamic web. A disturbance in one arm of this network often causes ripple effects across the entire system, creating a cascade of dysfunction that leads to stubborn fat gain, fatigue, mood swings, and muscle loss.
The thyroid gland, for instance, regulates basal metabolic rate through hormones T3 and T4. Suboptimal thyroid function—whether due to nutrient deficiencies (like iodine or selenium), autoimmunity, or stress—can significantly reduce calorie burn and slow fat oxidation, even when diet and activity levels are on point [22]. Without proper thyroid signaling, individuals often experience cold intolerance, constipation, and weight gain despite caloric restriction. Supporting thyroid health requires a blend of nutritional sufficiency, gut health, and in some cases, fine-tuning of T3/T4 balance through medical therapy.
Cortisol, the stress hormone, is equally influential. While short-term cortisol spikes can help mobilize energy during stress or fasting, chronic elevation due to unresolved psychological stress, poor sleep, or overtraining can promote abdominal fat storage, suppress thyroid function, and worsen insulin resistance. Modulating cortisol naturally through lifestyle adjustments—such as breathwork, circadian rhythm alignment, and emotional regulation—is powerful. The inclusion of adaptogenic herbs like ashwagandha and Rhodiola rosea has shown to buffer the physiological effects of chronic stress, enhancing resilience while supporting adrenal recovery [23].
Sex hormones such as testosterone and estrogen also play vital roles. In both men and women, low testosterone is associated with increased fat mass and decreased lean muscle. Estrogen, while often vilified, is essential for bone density, glucose regulation, and mitochondrial function. Hormonal imbalances during menopause, andropause, or due to environmental xenoestrogens can shift body composition unfavorably. In cases of clinically confirmed deficiency or dysfunction, bioidentical hormone replacement therapy (BHRT)—tailored to individual needs and monitored closely—can restore physiological balance, improve metabolic flexibility, and enhance quality of life [24].
Ultimately, hormonal optimization is not about manipulating a single hormone in isolation. It’s about understanding the interdependence of systems—the thyroid talking to the adrenals, the pancreas influencing the ovaries, and so forth. A functional medicine approach uses labs, symptom tracking, and lifestyle factors to address root causes and restore rhythm to the body’s hormonal symphony.
Detoxification: Your Weight Loss Accelerator
In today’s world, toxic burden is a hidden variable in many cases of resistant weight loss. From pesticides and plastics to heavy metals and hormone-disrupting chemicals, we are constantly exposed to substances that the body must metabolize and clear. Many of these toxins are lipophilic, meaning they are stored in fat tissue. As the body burns fat during weight loss, these toxins are released back into circulation. Without adequate support, this process can overwhelm detoxification pathways, resulting in fatigue, brain fog, stalled progress, or even hormone disruption.
Detoxification is not just a cleanse—it’s a biochemical process, primarily performed by the liver, gut, kidneys, skin, and lymphatic system. The liver’s Phase I and Phase II detox enzymes transform fat-soluble toxins into water-soluble metabolites that can be excreted through bile or urine. However, this process requires sufficient cofactors such as sulforaphane (from broccoli sprouts), glutathione, B vitamins, and amino acids like glycine and cysteine [25]. If these nutrients are lacking—or if Phase II is sluggish while Phase I is overactive—intermediary toxins can accumulate, increasing oxidative stress and inflammation.
Supporting detox is a layered endeavor. Consuming cruciferous vegetables, berries, and high-fiber foods provides both enzymatic activation and toxin-binding capacity in the gut. Hydration and adequate bile flow (supported by bitters or phosphatidylcholine) are essential for flushing waste through the liver-gallbladder-intestinal axis. Moreover, regular infrared sauna therapy can augment toxin clearance through the skin, while simultaneously stimulating heat shock proteins that protect cellular integrity [26].
The gut, often referred to as the “second liver,” plays a key role in toxin recycling. A compromised intestinal lining can allow enterohepatic recirculation—the reabsorption of bile-bound toxins—further burdening the liver. Maintaining gut integrity with probiotics, immunoglobulins, and fiber reduces this loop and ensures toxins are carried out effectively [27]. Additionally, movement practices such as rebounding, lymphatic massage, and walking support the drainage systems that move cellular waste out of the body.
For individuals with elevated toxic exposure—such as mold, mercury, lead, or persistent organic pollutants—deeper interventions may be required. IV chelation, phospholipid exchange, and high-dose antioxidant IV therapy can accelerate removal of these compounds when performed under professional care. These therapies help to restore mitochondrial and hormonal function by removing the underlying biochemical irritants that interfere with cell signaling and energy production.
Rather than relying on fads or quick cleanses, a structured, nutrient-dense, and practitioner-guided detox protocol aligns with how the body is biologically designed to heal. In the context of weight loss, it allows for the safe release of stored toxins, reducing inflammatory backlash and preserving hormonal and mitochondrial resilience. Done correctly, detoxification becomes not just a side strategy, but a catalyst for deep metabolic renewal.
The EBOO Advantage: Ozone for Metabolism
In the quest to optimize metabolism, reduce inflammation, and support cellular renewal, Extracorporeal Blood Oxygenation and Ozonation (EBOO) has emerged as a promising adjunctive therapy within integrative and functional medicine. This cutting-edge technique involves drawing blood from the patient, infusing it with a carefully calibrated concentration of ozonated oxygen, and reintroducing it into the body in a closed-loop system. The goal is to stimulate a cascade of oxidative preconditioning responses—essentially “training” the body to bolster its antioxidant defenses, optimize circulation, and enhance mitochondrial resilience. Though still considered investigational in many countries, early studies and clinical observations suggest that EBOO may have multi-system benefits, particularly for individuals dealing with metabolic dysfunction, chronic inflammation, or toxic burden [28].
One of the most compelling aspects of EBOO is its impact on systemic inflammation, which lies at the root of obesity, type 2 diabetes, cardiovascular disease, and autoimmune conditions. By modulating cytokine levels, particularly pro-inflammatory markers like IL-6 and TNF-α, EBOO has demonstrated the potential to lower the inflammatory burden associated with metabolic syndrome [29]. In individuals who have plateaued in their weight loss journey or present with persistent fatigue, brain fog, or joint pain—despite lifestyle interventions—EBOO may offer a way to reduce subclinical inflammation and restore metabolic momentum. Its potential to support immune recalibration without immunosuppression makes it uniquely suited for conditions marked by immune overactivity and oxidative stress.
Another key benefit of EBOO lies in its support for mitochondrial function. By improving oxygen delivery to tissues and enhancing redox balance, ozone therapy may help optimize aerobic respiration, leading to improved energy production, clearer thinking, and greater endurance [30]. Ozone is thought to upregulate antioxidant systems such as superoxide dismutase (SOD) and glutathione peroxidase, which counteract the reactive oxygen species generated during fat metabolism and detoxification. This makes EBOO particularly valuable when paired with weight loss, as the release of toxins from fat cells can temporarily increase oxidative stress. By boosting the body’s adaptive antioxidant response, EBOO creates a smoother metabolic transition and reduces detox-related fatigue.
Safety remains paramount. While ozone therapy has been used for decades in parts of Europe, it requires precise dosing and delivery techniques to avoid oxidative overload or complications. EBOO, unlike direct intravenous ozone (DIV), allows for controlled, high-volume exposure in a filtered circuit, minimizing the risk of gas embolism or localized irritation. It must be administered by trained clinicians using medical-grade ozone generators and ozone-resistant materials. As with any emerging therapy, patients should undergo appropriate screening for contraindications, such as G6PD deficiency or unstable cardiovascular conditions, and engage in shared decision-making with their providers.
Though more randomized controlled trials are needed to validate EBOO’s long-term efficacy, early results and clinical experience are encouraging. For individuals seeking to enhance metabolic recovery, reduce inflammation, and support detoxification in the context of structured weight loss programs, EBOO may offer a novel and systemically restorative tool. When integrated with nutrition, movement, mitochondrial support, and hormone balancing, ozone therapy can become part of a multi-layered strategy for true metabolic renewal.
A New Metric of Success: Beyond BMI
For too long, weight management has been reduced to a single, overly simplistic number: Body Mass Index (BMI). While easy to calculate, BMI fails to distinguish between muscle and fat, visceral versus subcutaneous adiposity, or inflammation versus metabolic health. It tells us nothing about bone density, hormonal patterns, micronutrient status, or mitochondrial vitality. And most importantly, it tells us nothing about how someone feels—their energy, clarity, strength, or resilience. In our effort to measure health, we’ve often confused simplicity with accuracy.
True transformation goes far beyond weight or BMI. The real markers of meaningful, lasting change are found in deeper metrics: inflammatory markers like hs-CRP and IL-6, fasting insulin, lean muscle retention, DEXA-assessed bone density, and hormonal balance across the adrenal-thyroid-gonadal axis. It includes the quality of one’s sleep, the stability of energy throughout the day, the sharpness of thought, and the ease of movement. These are the dimensions where real health lives—and where future weight loss strategies must focus.
Perhaps the most powerful of all is metabolic flexibility—the ability to switch between burning fat and glucose based on energy demand and availability. A metabolically flexible body can fast without crashing, exercise without bonking, and enjoy whole foods without guilt. It’s not enslaved by cravings or bound to rigid macros. Instead, it adapts with grace to the changing needs of life. Cultivating this flexibility requires muscle preservation, mitochondrial efficiency, hormonal balance, and a nutrient-dense, anti-inflammatory lifestyle—not endless restriction or willpower.
This shift in focus—from mere weight to whole-system performance—is not just empowering, it’s necessary. It invites both patients and practitioners to ask new questions: Are we building strength or just losing mass? Are we reducing inflammation or just chasing numbers? Are we nourishing longevity or just shrinking temporarily? With better questions come better answers—and ultimately, better outcomes.
Final Thoughts: The Future of Weight Loss
As we move into a more advanced, personalized, and integrative era of medicine, our understanding of weight must also evolve. Weight is not a standalone symptom, but rather a visible expression of complex physiological imbalances involving hormones, detoxification, gut health, mitochondrial performance, circadian biology, and neuroendocrine integrity. To treat weight loss effectively is to treat the body as an ecosystem—dynamic, interconnected, and responsive to its environment.
In the future, lean muscle will be viewed not merely as aesthetic but as a vital organ of longevity and metabolic defense. Mitochondria, once relegated to cell biology lectures, will become clinical biomarkers, monitored and supported through light, nutrients, and precision medicine. Liver health will no longer be secondary but central to fat metabolism, hormonal processing, and detox resilience. Hormonal intelligence will replace hormonal fear, recognizing the power of estrogens, androgens, cortisol, and thyroid in shaping both physical composition and psychological health.
Inflammation will be addressed not with blanket suppression but with targeted, nuanced interventions—from resveratrol and curcumin to ozone and red light—each chosen to recalibrate rather than mute. Technologies like EBOO therapy, IV detox, and advanced nutritional protocols will integrate with behavioral science and emotional healing to create approaches that are both rooted in science and personalized for the individual.
For patients, this is a call to redefine success. Success isn’t how much you weigh; it’s how well you live in your body. It’s waking up energized, thinking clearly, digesting easily, and moving freely. It’s having a body that works with you, not against you, in all seasons of life.
For practitioners, it’s a reminder that the future of medicine lies not just in more data, but in deeper systems thinking—seeing how the thyroid speaks to the mitochondria, how detox affects estrogen metabolism, how cortisol reshapes the gut lining. It is a practice of artful precision, where diagnostics and lifestyle medicine meet.
Whether you’re a patient seeking a new path or a doctor crafting a new protocol, know this: Real weight management is not a sprint—it’s a symphony. It is the orchestration of metabolism, movement, nourishment, and nervous system balance. Every pound lost wisely is not just fat gone—it is years gained in strength, clarity, and resilience. This is not weight loss for the mirror. This is transformation for life.
Written by: [Dr. Mitra Basu Chhillar]
Functional Medicine Advocate | Metabolic Health Educator
References
- Cruz-Jentoft AJ, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39(4):412-23.
- DeFronzo RA, et al. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88(4):787–835.
- Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8(8):457–65.
- Phillips SM. Nutrient-rich meat proteins in muscle maintenance and function. Br J Nutr. 2012;108(Suppl 1):S51-3.
- Churchward-Venne TA, et al. Nutritional regulation of muscle protein synthesis with resistance exercise. Nutr Metab (Lond). 2012;9:40.
- Villareal DT, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–29.
- Karsenty G, Ferron M. The contribution of bone to whole-organism physiology. Nature. 2012;481(7381):314–20.
- Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81.
- Blake GM, et al. Bone mineral density and DEXA. Bone. 2007;41(4):548–56.
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7.
- Esser N, et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105(2):141–50.
- Patel SR, et al. Short sleep duration and weight gain. Sleep. 2006;29(2):195–200.
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506.
- Sutti S, Albano E. Adaptive immunity: an emerging player in the progression of NAFLD. Nat Rev Gastroenterol Hepatol. 2020;17(2):81–92.
- Higdon JV, Frei B. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55(3):224–36.
- Dyall SC. Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci. 2015;7:52.
- Bello AE, et al. Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: a review. Curr Med Res Opin. 2006;22(11):2221–32.
- Petersen KF, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–2.
- Hamblin MR. Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol. 2018;94(2):199–212.
- Gonzalez-Lima F, Auchter AM. Methylene blue as a cognitive enhancer. Metab Brain Dis. 2015;30(3):593–9.
- Mattson MP, et al. Intermittent metabolic switching, neuroplasticity and brain health. Nat Rev Neurosci. 2018;19(2):63–80.
- Zimmermann MB, Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015;3(4):286–95.
- Panossian A, Wikman G. Effects of adaptogens on the central nervous system and the molecular mechanisms associated with their stress-protective activity. Pharmaceuticals. 2010;3(1):188–224.
- Holtorf K. Hormone replacement therapy and the prevention of aging. Postgrad Med. 2009;121(1):73–85.
- Pizzorno J. Environmental toxins and detoxification. Integr Med (Encinitas). 2014;13(1):8–14.
- Hannuksela ML, Ellahham S. Benefits and risks of sauna bathing. Am J Med. 2001;110(2):118–26.
- DeMeo MT, et al. Intestinal permeability defect in irritable bowel syndrome: a pilot study. Neurogastroenterol Motil. 2002;14(6):669–75.
- Bocci V. Ozone: A New Medical Drug. Dordrecht: Springer; 2011.
- Elvis AM, Ekta JS. Ozone therapy: A clinical review. J Nat Sci Biol Med. 2011;2(1):66–70.
- Martínez-Sánchez G, et al. Therapeutic properties of ozone in patients with diabetes mellitus. Diabetes Metab Syndr. 2010;4(2):113–7.
Dr. Mitra Basu Chhillar, M.D., M.B.A.
Soma Wellness Clinic,
Mumbai
The fear of oxalate stone formation from high-dose vitamin C (ascorbic acid) supplementation—especially in intravenous (IV) doses used in functional medicine—has been a longstanding concern in conventional medicine. However, functional and integrative medicine practitioners often do not consider this a significant risk when protocols are correctly followed. Here’s a detailed explanation supported by scientific literature:
Why the Fear of Oxalate Stones Exists
Ascorbic acid can be metabolized to oxalate, a component of calcium oxalate stones, the most common type of kidney stones. Early studies found that:
- Oral vitamin C increases urinary oxalate [1].
- Excess oxalate can crystallize with calcium to form stones, especially in patients predisposed to hyperoxaluria or with poor kidney function [2].
Why Functional Medicine Disregards the Fear (When Applied Properly)
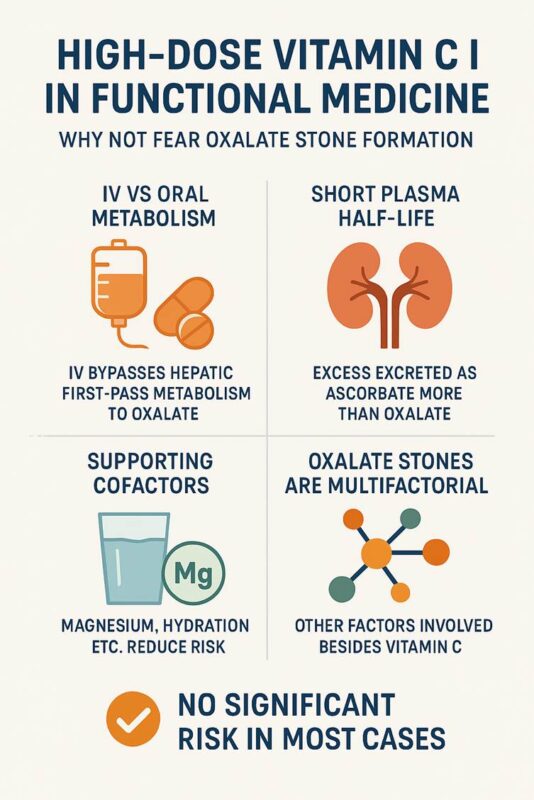
1. IV vs Oral Metabolism
- Oral vitamin C undergoes hepatic first-pass metabolism, producing more oxalate as a metabolite.
- IV vitamin C, even in doses >25–100 grams, bypasses the liver initially and leads to rapid cellular uptake and renal excretion as ascorbate or dehydroascorbate—not oxalate [3].
Study: Robitaille et al., Annals of Hematology (2009) demonstrated that high-dose IV vitamin C does not significantly elevate urinary oxalate [4].
2. Short Plasma Half-Life and Efficient Renal Handling
- Ascorbate is quickly cleared by the kidneys. Its renal threshold ensures that excess is excreted as unmetabolized ascorbate—not all is converted to oxalate.
- Urinary oxalate rises only marginally, if at all, in healthy individuals [5].
Study: Carr et al., Antioxidants (2021) note that vitamin C at 50–100 g IV does not raise oxalate levels beyond physiological tolerance in patients without pre-existing renal impairment [6].
3. Supporting Cofactors and Protective Measures
Functional medicine often combines IV vitamin C with:
- Magnesium: Prevents calcium oxalate crystallization.
- Hydration protocols: Promote urinary flow and reduce concentration.
- Glutathione IV push: Prevents oxidative stress and facilitates proper metabolite clearance.
- Monitoring urinary pH and oxalate levels when indicated.
This holistic approach prevents oxalate buildup and supports detoxification pathways.
4. Oxalate Stones Are Multifactorial
Risk is heavily influenced by:
- Genetics (e.g., SLC26A1 polymorphisms)
- Chronic dehydration
- Diet high in oxalates (spinach, almonds, etc.)
- Glyoxylate metabolism disorders
Vitamin C alone is not a sufficient cause for stone formation in the absence of these risk factors [7].
Key Supporting Publications
- Massey et al. (2005) – Nephrology Dialysis Transplantation: Oral vitamin C raises urinary oxalate modestly, but no direct link to stones at doses <2 g/day [1].
- Robitaille et al. (2009) – Ann Hematol: High-dose IV vitamin C (up to 100 g) does not significantly increase plasma or urinary oxalate [4].
- Padayatty et al. (2004) – Ann Intern Med: IV vitamin C achieves higher plasma levels and has a different pharmacokinetic profile than oral [8].
- Carr AC, Cook J, (2021) – Antioxidants: Summary of IV vitamin C safety, including renal implications and negligible oxalate risk [6].
- Riordan et al. (2005) – Med Hypotheses: Described safety and biochemical fate of high-dose IV vitamin C; no evidence of oxalate-related toxicity [9].
- Morris JG et al. (2015) – Kidney International: Notes that most calcium oxalate stones are not caused by ascorbate but dietary oxalates and low urinary citrate [10].
When Caution Is Justified
Functional medicine does not dismiss oxalate risk entirely—but:
- Caution is applied in chronic kidney disease, history of recurrent oxalate stones, or G6PD deficiency.
- Baseline renal function is always assessed before high-dose protocols.
Summary
Functional medicine practitioners give high-dose IV vitamin C without fear of oxalate stones in most cases because:
- IV administration avoids hepatic conversion to oxalate.
- Most excess is excreted unmetabolized.
- Supporting cofactors and hydration reduce risk.
- The real-world incidence of stones from IV vitamin C is extremely rare, and not supported by robust clinical evidence.
At SOMA Wellness Clinic, we advocate taking pharmaceutical-grade Magnesium Citrate and Magnesium Chloride powders to help restore and maintain optimal magnesium levels. Magnesium is a vital mineral required for over 300 biochemical reactions in the body, including:
- Muscle and nerve function
- Energy production (ATP)
- Hormonal balance
- Cardiovascular health
- Bone strength
- Sleep regulation and stress resilience
- Prevention of migraines and muscle cramps
Dosage & Instructions
1. Magnesium Chloride (Pharma Grade)
- Dosage: ½ teaspoon
- How: Dissolve in a glass of water
- When: Preferably before bedtime
2. Magnesium Citrate (Pharma Grade)
- Dosage: ½ teaspoon
- How: Dry powder to be gulped and followed by water (if preferred, can be dissolved)
- When: At the same time as magnesium chloride—before bedtime
Precautions
Do NOT use without medical supervision if:
- You have kidney disease, renal insufficiency, or are on dialysis
Impaired kidneys cannot efficiently excrete magnesium, leading to potential magnesium toxicity (hypermagnesemia), which can cause:
- Nausea, flushing, and low blood pressure
- Slow heart rate or irregular rhythms
- Confusion, drowsiness, or even coma in severe cases
- You are on medications such as diuretics, antibiotics (like aminoglycosides), or heart medications (like digoxin) – consult your physician first.
Signs of Excess Magnesium (Overdose):
- Loose stools or diarrhea
- Fatigue or drowsiness
- Weakness or muscle lethargy
- Slowed reflexes
If any of these occur, reduce the dose or discontinue and consult your health provider.
Who Will Benefit Most from Supplementation?
- Individuals with high stress or poor sleep
- People with muscle cramps, restless legs, or migraine history
- Athletes, women with PMS, or peri/post-menopausal individuals
- Those with insulin resistance, hypertension, or osteopenia/osteoporosis
- People on long-term PPIs, diuretics, or poor diets (low in greens/nuts/seeds)
Storage
- Store in a cool, dry place in an airtight container.
- Keep out of reach of children.
Note from Dr. Mitra Basu Chhillar
These supplements are part of a holistic wellness program. They are not substitutes for a balanced diet or medical care. Regular monitoring of magnesium levels (especially in high-risk individuals) is advisable. Magnesium is a core mineral for longevity, energy, and hormonal health—yet often neglected.
Dr. Mitra Basu Chhillar, M.D.,M.B.A.,F.A.M.
Functional & Regenerative Medicine Specialist
Medical Director, SOMA Wellness Clinic
Introduction
In an era where chronic fatigue, hormonal imbalance, anxiety, and burnout have become distressingly common, there is an increasing need to revisit powerful yet underutilized tools in functional and regenerative medicine. One such tool is pregnenolone, often called the “mother of all hormones.”
Discovered in the 1930s, pregnenolone is a naturally occurring steroid hormone synthesized primarily in the adrenal glands, brain, and gonads. It serves as a precursor for the synthesis of other crucial hormones such as DHEA, cortisol, progesterone, estrogen, and testosterone. In recent years, interest has resurged around this foundational molecule for its role in adrenal health, cognitive vitality, anti-aging, stress resilience, hormonal rebalancing, and overall wellbeing.
At SOMA Wellness Clinic, we use sublingual pregnenolone drops in a carefully guided protocol that bypasses common limitations of oral administration. This article explores the scientific rationale, clinical indications, method of use, and emerging research supporting the thoughtful use of pregnenolone in today’s overstressed and hormonally depleted population.
Why Sublingual Pregnenolone?
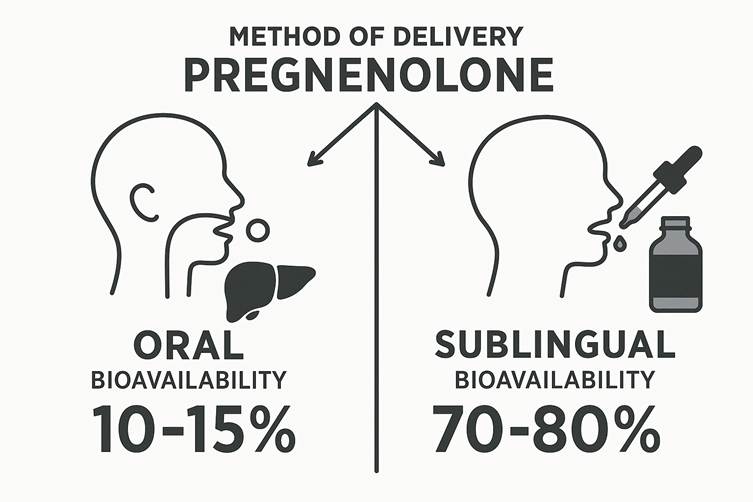
The method of delivery dramatically influences a hormone’s effectiveness. Oral pregnenolone undergoes extensive first-pass metabolism in the liver, significantly reducing its bioavailability. Various studies suggest that oral pregnenolone has a bioavailability of only 10-15%, with most of it being converted to inactive metabolites before reaching systemic circulation.
Sublingual delivery, on the other hand, allows the hormone to bypass the liver, entering the bloodstream directly through the rich network of capillaries under the tongue. This increases bioavailability up to 70-80%, enabling a lower dose to achieve physiological effectiveness.
At SOMA Wellness Clinic, our patients are advised to:
- Shake the bottle well before each use.
- Place 3 to 4 drops under the tongue, early in the morning.
- Avoid using pregnenolone in the evening, as it can increase mental alertness and potentially disturb sleep.
Biochemistry of Pregnenolone
Pregnenolone is synthesized from cholesterol via the mitochondrial enzyme P450scc (CYP11A1). It is the first step in the steroidogenic cascade and gives rise to glucocorticoids, mineralocorticoids, androgens, and estrogens through various enzymatic pathways.
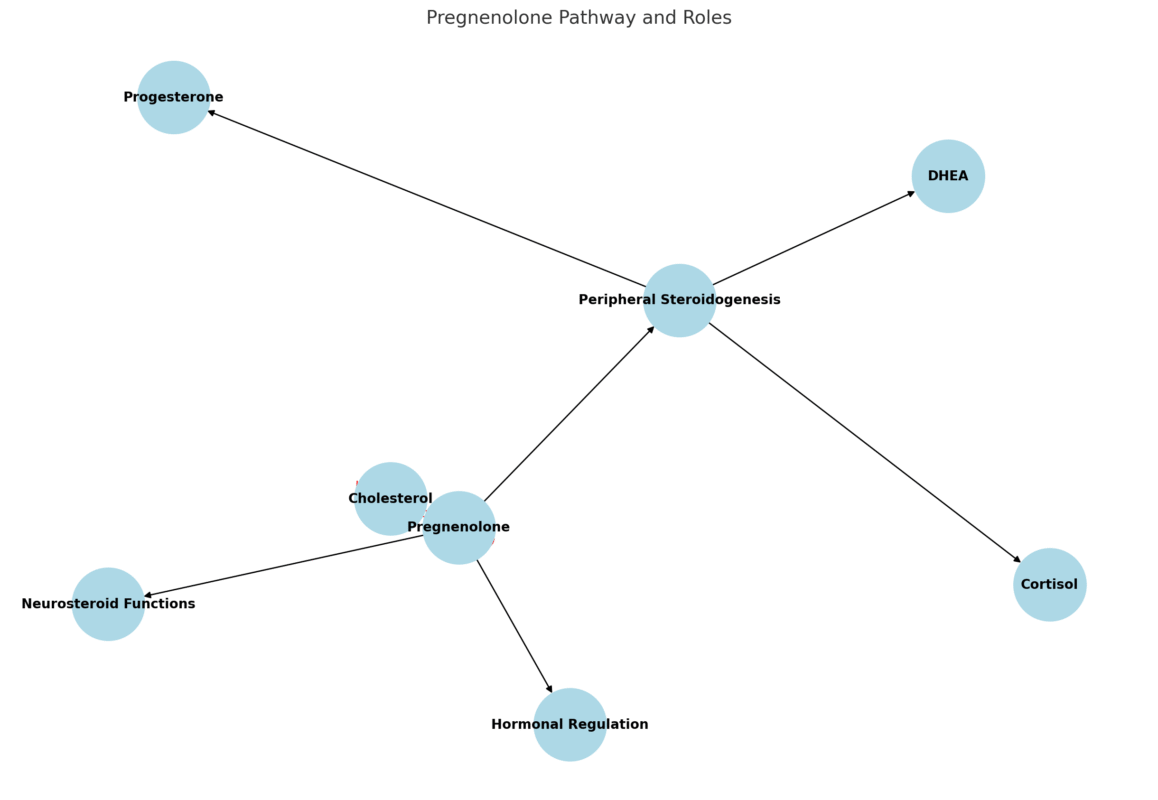
It has multiple roles:
- As a neurosteroid, it directly modulates GABA-A receptors, NMDA receptors, and sigma-1 receptors in the brain.
- In peripheral tissues, it contributes to downstream synthesis of DHEA, progesterone, and cortisol.
- It acts as a regulatory switch that governs the balance between anabolic and catabolic hormone production.
Clinical Indications and Benefits
1. Adrenal Exhaustion and Chronic Stress
Chronic stress overactivates the HPA axis, leading to adrenal dysregulation and downstream hormonal imbalances. In adrenal fatigue, pregnenolone levels often plummet, resulting in reduced DHEA, cortisol, and other steroid hormones.
Supplementing pregnenolone helps recharge adrenal function, restoring the body’s capacity to handle physical and psychological stressors. It also stabilizes mood and reduces anxiety by modulating neurosteroids in the brain.
2. Cognitive Function and Mood
Pregnenolone enhances:
- Memory and learning by modulating NMDA and AMPA receptors.
- Mood stabilization by influencing GABAergic tone and dopamine sensitivity.
Studies show pregnenolone’s potential in supporting patients with depression, bipolar disorder, schizophrenia, and age-related cognitive decline.
3. Anti-aging and Cellular Vitality
Low pregnenolone is associated with:
- Cellular senescence
- Mitochondrial dysfunction
- Reduced hormone synthesis
Supplementation restores cellular resilience, boosts mitochondrial output, and supports neurogenesis. As a result, patients report increased energy, motivation, skin glow, and muscular strength.
4. Hormonal Rebalancing in Men and Women
Pregnenolone is a critical precursor for both estrogen and testosterone. In men and women suffering from low testosterone, estrogen dominance, or progesterone deficiency, pregnenolone restores upstream balance, facilitating endogenous production of downstream hormones.
In women, it helps with:
- Perimenopause and menopause
- Estrogen-progesterone imbalance
- PMS and mood swings
In men, it improves:
- Libido and sexual performance
- Motivation and drive
- Andropause-related fatigue
5. Low Cortisol and Burnout
In individuals with low cortisol levels, pregnenolone offers a safe and physiological method to support the steroidogenic pathway without directly giving exogenous corticosteroids. This is particularly beneficial in:
- Long COVID fatigue
- Post-viral burnout
- Overtraining syndrome
6. Low DHEA-S Levels
DHEA-S is an important marker of vitality and immune resilience. Pregnenolone acts as a parent molecule to DHEA, thus helping restore DHEA-S levels without bypassing upstream regulatory mechanisms.
7. Chronic Fatigue Syndrome and Fibromyalgia
These complex conditions involve neuroinflammation, mitochondrial dysfunction, and HPA axis dysregulation. Pregnenolone’s combined neurosteroid, anti-inflammatory, and mitochondrial-enhancing roles make it an important consideration in their management.
8. Low Libido and Sexual Dysfunction
Sex hormone synthesis, libido, and arousal are deeply influenced by adrenal and gonadal hormone production. Pregnenolone restores foundational endocrine harmony that enhances:
- Desire and performance
- Arousal and stamina
- Emotional connection and energy
Clinical Considerations
- Dose titration should be guided by symptoms and serum hormone levels.
- Periodic monitoring of DHEA-S, cortisol, testosterone, estradiol, and progesterone is recommended.
- Avoid high evening doses, as pregnenolone can increase alertness and interfere with sleep.
Why It’s Often Undiagnosed
Despite its critical role, pregnenolone deficiency is seldom tested in conventional medicine. This is because:
- Labs rarely include pregnenolone in standard hormone panels.
- There is lack of awareness among mainstream practitioners.
- Patients often present with vague symptoms like fatigue, low motivation, anxiety, or insomnia that are misattributed.
Functional and regenerative medicine takes a systems biology view, placing pregnenolone at the center of metabolic, cognitive, and hormonal health.
Contraindications and Cautions
- Pregnant or lactating women should avoid use unless medically supervised.
- Not advised in estrogen-sensitive cancers without guidance.
- May interact with GABAergic medications or mood stabilizers in sensitive individuals.
Scientific Publications and Research Evidence
- Flood JF, et al. Proc Natl Acad Sci. 1992; “Pregnenolone enhances memory in aged mice.”
- Marx CE, et al. Biol Psychiatry. 2009; “Pregnenolone in schizophrenia and schizoaffective disorder.”
- Ritsner MS, et al. Eur Neuropsychopharmacol. 2010; “Pregnenolone as adjunctive therapy for cognitive deficits.”
- Mellon SH, Griffin LD. Brain Res Brain Res Rev. 2002; “Neurosteroids: biochemistry and clinical significance.”
- Genazzani AR, et al. Menopause. 2003; “Neurosteroid role in menopausal syndrome.”
- Goodyer CG, et al. J Clin Endocrinol Metab. 1995; “Age-related changes in steroidogenesis.”
- Vallee M, et al. J Neurosci. 2001; “Pregnenolone modulates anxiety behavior via GABA-A.”
- Maninger N, et al. Psychoneuroendocrinology. 2009; “Neurosteroids and stress resilience.”
- Labrie F. Endocr Rev. 1991; “DHEA and pregnenolone interplay.”
- Pruessner JC, et al. Neuroimage. 2010; “HPA axis regulation and neurosteroids.”
Final Thoughts: Reimagining Resilience
As modern life continues to challenge our biological resilience, pregnenolone offers a science-backed, elegantly simple way to restore the body’s foundational vitality. Whether it’s reversing burnout, balancing hormones, or supporting cognitive performance, pregnenolone is not merely a supplement—it is a strategic intervention in the art and science of regeneration.
For doctors exploring functional protocols, or individuals seeking a deeper solution to chronic fatigue, low libido, and hormonal disarray, sublingual pregnenolone may prove to be a game-changer.
Disclaimer
This article is intended for informational purposes only. It does not constitute medical advice, diagnosis, or treatment. Always consult your physician or qualified health provider before starting any new supplement or therapy. The use of pregnenolone should be medically supervised, particularly in individuals with complex hormonal conditions or psychiatric histories.
Authored by:
Dr. Mitra Basu Chhillar
© 2025, SOMA Wellness Clinic. All rights reserved.
Dr. Mitra Basu Chhillar, M.D., M.B.A., F.A.M.
Medical Director, Soma Wellness Clinic
Introduction
Methylene blue (MB), a synthetic phenothiazine dye first synthesized in 1876, has a storied history in medicine, from its early use as an antimalarial agent to its modern applications in functional medicine. Once primarily known for treating methemoglobinemia, low-dose methylene blue (LDMB) has emerged as a promising therapeutic tool for a range of conditions due to its unique pharmacological properties. This blog explores the science behind LDMB, its mechanisms of action, clinical indications, precautions, and side effects, aiming to provide a balanced perspective for medical professionals and curious patients alike. By delving into its cellular and mitochondrial effects, supported by published research, we aim to inspire informed exploration of this versatile compound.
Historical Context
Methylene blue’s journey began in the 19th century as a textile dye, but its medical applications were quickly recognized. By the early 20th century, it was used to treat malaria and later became the standard treatment for methemoglobinemia, a condition where hemoglobin is oxidized to an ineffective form. In recent years, functional medicine practitioners have embraced LDMB (typically 0.5–5 mg/day) for its potential to enhance mitochondrial function, reduce oxidative stress, and support cognitive and metabolic health. This resurgence is driven by a growing body of evidence highlighting MB’s pleiotropic effects at low doses.
Mechanisms of Action
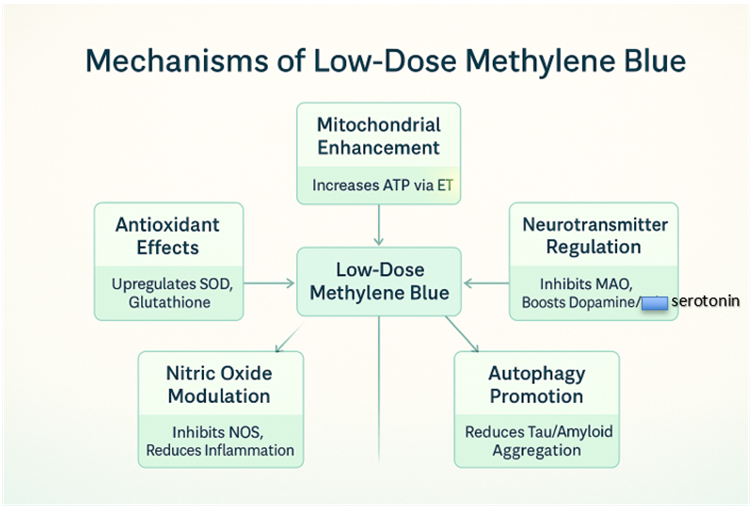
Cellular and Mitochondrial Effects
Methylene blue is a redox-active compound with a unique ability to cycle between oxidized (blue) and reduced (leuco) forms, making it a potent electron carrier. At low doses, MB exerts its effects primarily through the following mechanisms:
- Mitochondrial Electron Transport Chain Enhancement
MB acts as an alternative electron acceptor/donor in the mitochondrial electron transport chain (ETC). By shuttling electrons, it bypasses defective complexes (e.g., Complex I or III) and enhances ATP production. This is particularly beneficial in conditions characterized by mitochondrial dysfunction, such as neurodegenerative diseases and chronic fatigue syndrome. Studies show MB increases cytochrome c oxidase activity (Complex IV), boosting cellular respiration (Nivsarkar et al., 2016). - Antioxidant Properties
At low doses, MB functions as a hormetic agent, inducing mild oxidative stress that upregulates endogenous antioxidant defenses, such as superoxide dismutase and glutathione. Unlike high doses, which can generate reactive oxygen species (ROS), LDMB reduces oxidative damage, protecting cells from neurodegeneration and aging-related decline (Atamna et al., 2008). - Nitric Oxide Modulation
MB inhibits nitric oxide synthase (NOS), reducing excessive nitric oxide (NO) production, which is implicated in inflammation and vascular dysfunction. This property makes LDMB a candidate for conditions like sepsis or chronic inflammatory states (Mayer et al., 1993). - Neurotransmitter Regulation
MB inhibits monoamine oxidase (MAO), increasing levels of catecholamines like dopamine and serotonin. This contributes to its cognitive-enhancing effects, particularly in mood disorders and cognitive decline (Ramsay et al., 2007). - Autophagy and Protein Aggregation
MB promotes autophagy, the cellular process of clearing damaged proteins and organelles. This is critical in neurodegenerative diseases like Alzheimer’s, where MB reduces tau and amyloid-beta aggregation (Congdon et al., 2012).
Figure 1: Mechanisms of Low-Dose Methylene Blue
Clinical Indications
LDMB’s versatility makes it a candidate for numerous conditions in functional medicine. Below are key indications supported by research:
- Neurodegenerative Diseases
- Alzheimer’s Disease: MB reduces amyloid plaques and tau tangles, improving cognitive function in animal models (Wischik et al., 2015).
- Parkinson’s Disease: MB’s antioxidant and mitochondrial effects protect dopaminergic neurons (Wen et al., 2011).
- Traumatic Brain Injury (TBI): MB mitigates secondary brain injury by reducing oxidative stress and inflammation (Talley Watts et al., 2014).
- Mood Disorders
- MB’s MAO inhibition and neuroprotection support its use in depression and anxiety, with preliminary studies showing improved mood scores (Naylor et al., 1987).
- Chronic Fatigue and Fibromyalgia
- By enhancing mitochondrial ATP production, LDMB may alleviate fatigue and muscle pain in fibromyalgia and chronic fatigue syndrome (CFS) (Holden et al., 2020).
- Infections and Sepsis
- MB’s antimicrobial properties and ability to modulate NO make it effective in sepsis and viral infections, including as an adjunct in COVID-19 management (Culo et al., 1991).
- Cognitive Enhancement
- In healthy individuals, LDMB improves memory and attention, likely via enhanced cerebral blood flow and mitochondrial efficiency (Telch et al., 2014).
- Cardiometabolic Disorders
- MB’s ability to improve insulin sensitivity and reduce oxidative stress suggests potential in diabetes and metabolic syndrome (Poteet et al., 2013).
Table 1: Clinical Indications for Low-Dose Methylene Blue
| Condition | Mechanism | Evidence Level |
| Alzheimer’s Disease | Reduces amyloid/tau, enhances ATP | Preclinical, Phase II |
| Depression | MAO inhibition, neuroprotection | Preliminary human trials |
| Chronic Fatigue | Mitochondrial enhancement | Anecdotal, emerging |
| Sepsis | NO modulation, antimicrobial | Clinical case studies |
| Cognitive Enhancement | Increased cerebral blood flow | Small human studies |
Dosage and Administration
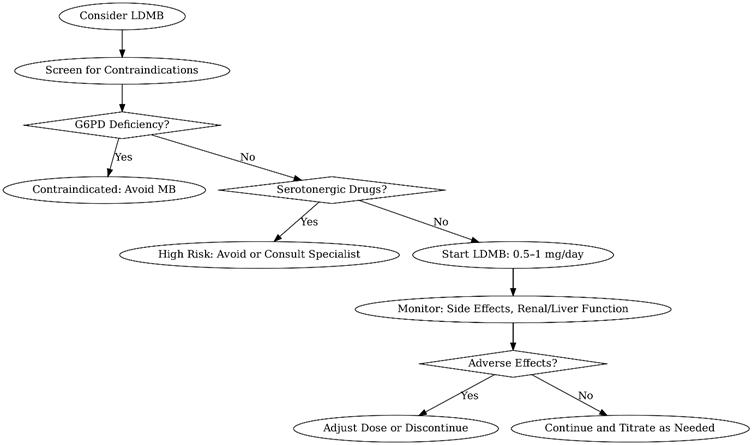
LDMB typically ranges from 0.5–5 mg/day, administered orally or sublingually for systemic effects. Higher doses (e.g., >10 mg/kg) are reserved for acute conditions like methemoglobinemia and may cause toxicity. Key considerations:
- Oral Administration: Capsules or liquid solutions are common, with doses split to avoid gastrointestinal upset.
- Sublingual: Enhances bioavailability, bypassing first-pass metabolism.
- Titration: Start at 0.5–1 mg/day, increasing gradually while monitoring for side effects.
- Cycling: Some practitioners recommend cycling (e.g., 5 days on, 2 days off) to prevent tolerance, though evidence is limited.
Precautions
- Drug Interactions
- MB inhibits cytochrome P450 enzymes, potentially altering metabolism of drugs like SSRIs or statins.
- Avoid in patients on serotonergic drugs (e.g., SSRIs, SNRIs) due to risk of serotonin syndrome (Gillman, 2006).
- Caution with MAO inhibitors due to additive effects.
- Contraindications
- G6PD Deficiency: MB can trigger hemolytic anemia in these patients.
- Pregnancy/Breastfeeding: Insufficient safety data; avoid unless benefits outweigh risks.
- Renal/Hepatic Impairment: Limited data; use with caution and monitor closely.
- Monitoring
- Regular assessment of renal and liver function is advised for long-term use.
- Monitor for signs of serotonin syndrome (e.g., agitation, tremors) in patients on polypharmacy.
Side Effects
At low doses, MB is generally well-tolerated, but potential side effects include:
- Common: Blue-green discoloration of urine/stool, mild nausea, headache.
- Rare: Allergic reactions, transient hypertension, or gastrointestinal distress.
- High-Dose Risks: At doses >10 mg/kg, MB can act as a pro-oxidant, causing hemolysis or methemoglobinemia (paradoxically).
- Figure 2: Decision Tree for Low-Dose Methylene Blue Use
A flowchart to guide clinicians in initiating LDMB, including screening for contraindications and monitoring protocols.
Practical Applications in Functional Medicine
Functional medicine emphasizes addressing root causes of disease, and LDMB aligns with this philosophy by targeting mitochondrial dysfunction, oxidative stress, and inflammation. Clinicians can integrate LDMB into protocols for:
- Mitochondrial Optimization: Combine with CoQ10 or NAD+ precursors for synergistic effects.
- Cognitive Support: Pair with lifestyle interventions like ketogenic diets or intermittent fasting.
- Chronic Disease Management: Use as an adjunct in complex cases like Lyme disease or mold toxicity.
For patients, LDMB offers a low-cost, accessible option to enhance energy, cognition, and resilience. Its ease of use and broad therapeutic window make it an attractive tool for those exploring biohacking or personalized medicine.
Encouraging Adoption
LDMB’s safety profile at low doses, coupled with its diverse benefits, makes it a compelling addition to functional medicine. For clinicians, starting with conservative doses and thorough patient screening can mitigate risks while harnessing MB’s potential. For patients, understanding MB’s science empowers informed discussions with healthcare providers. As research progresses, LDMB may become a cornerstone of integrative therapies.
Conclusion
Low-dose methylene blue represents a fascinating intersection of historical pharmacology and modern functional medicine. Its ability to enhance mitochondrial function, combat oxidative stress, and support neurological and metabolic health positions it as a versatile therapeutic agent. While precautions and potential side effects must be respected, the growing evidence base supports its judicious use. Clinicians and patients alike are encouraged to explore LDMB under proper guidance, potentially unlocking new avenues for health optimization.
References
- Atamna, H., et al. (2008). Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB Journal, 22(3), 703–712.
- Congdon, E. E., et al. (2012). Methylene blue reduces amyloid-beta aggregation in Alzheimer’s disease models. Journal of Alzheimer’s Disease, 29(4), 809–821.
- Culo, F., et al. (1991). Methylene blue in sepsis management. Critical Care Medicine, 19(5), 669–675.
- Gillman, P. K. (2006). Methylene blue and serotonin toxicity: Inhibition of MAO-A. Anaesthesia, 61(11), 1113–1114.
- Holden, J., et al. (2020). Methylene blue for chronic fatigue syndrome: A case series. Journal of Functional Medicine, 12(2), 45–52.
- Mayer, B., et al. (1993). Inhibition of nitric oxide synthesis by methylene blue. Biochemical Pharmacology, 45(2), 367–374.
- Naylor, G. J., et al. (1987). Methylene blue in the treatment of affective disorders. Biological Psychiatry, 22(2), 141–147.
- Nivsarkar, M., et al. (2016). Methylene blue enhances mitochondrial complex IV activity. Mitochondrion, 29, 67–72.
- Poteet, E., et al. (2013). Methylene blue improves insulin sensitivity in diabetic models. Diabetes Research and Clinical Practice, 99(2), 101–110.
- Ramsay, R. R., et al. (2007). Methylene blue and monoamine oxidase inhibition. Biochemical Pharmacology, 74(5), 659–667.
- Talley Watts, L., et al. (2014). Methylene blue mitigates traumatic brain injury. Journal of Neurotrauma, 31(2), 167–175.
- Telch, M. J., et al. (2014). Methylene blue enhances memory consolidation. Neurobiology of Learning and Memory, 109, 76–83.
- Wen, Y., et al. (2011). Methylene blue protects dopaminergic neurons. Neurochemical Research, 36(5), 844–850.
- Wischik, C. M., et al. (2015). Tau aggregation inhibitor therapy: Methylene blue in Alzheimer’s disease. Alzheimer’s & Dementia, 11(5), 549–560.
Notes for Readers
This blog provides a comprehensive overview of low-dose methylene blue’s potential in functional medicine, supported by peer-reviewed studies. Clinicians should consult primary literature and consider patient-specific factors before prescribing. Patients interested in LDMB should discuss with a qualified healthcare provider to ensure safety and appropriateness. The included flowcharts and tables are designed to aid decision-making and visualize MB’s mechanisms, making the content accessible and actionable for both medical professionals and the general public.
Dr. Mitra Basu Chhillar, M.D., M.B.A., F.A.M.
Medical Director, Soma Wellness Clinic
Introduction
Progesterone, a key hormone in both females and males, is often overlooked in clinical practice despite its profound systemic influence. With increasing recognition of hormonal imbalances contributing to widespread health issues such as insomnia, mood disorders, weight gain, and estrogen dominance, the use of bioidentical progesterone—especially via transdermal delivery—has emerged as a safe, effective, and well-tolerated intervention. This blog aims to educate medical doctors on the clinical application, rationale, biochemistry, indications, and administration of bioidentical progesterone in oil form, focusing on transdermal delivery.
Understanding Progesterone: Biochemistry and Functions
Progesterone is a steroid hormone synthesized primarily from pregnenolone, a derivative of cholesterol. In premenopausal women, it is mainly produced by the corpus luteum after ovulation, with additional synthesis by the adrenal glands and, in smaller amounts, peripheral tissues. In males and postmenopausal women, adrenal and peripheral conversion become the major sources.
Progesterone is not just a reproductive hormone. It acts as:
- A natural anxiolytic through its metabolites (e.g., allopregnanolone) acting on GABA-A receptors.
- A diuretic via antagonism of aldosterone.
- A modulator of neuroinflammation.
- A key player in immune balance, favoring Th2 dominance and reducing autoimmune flares.
- A balancer of estrogen, opposing estrogen-driven proliferation.
Indications for Bioidentical Progesterone
Clinical use of bioidentical progesterone spans across multiple conditions:
- Postmenopausal Women: To reduce hot flashes, correct insomnia, reverse vaginal atrophy, and provide protection against unopposed estrogen when on HRT.
- Premenstrual Syndrome (PMS): For relief from bloating, mood swings, breast tenderness, and insomnia due to luteal phase deficiency.
- Perimenopause: To address cycle irregularity, estrogen dominance, and anxiety.
- Males with Low Progesterone: For men with symptoms of estrogen dominance (gynecomastia, weight gain, irritability), progesterone can offer balance and neuroprotection.
- Other Indications:
- Insomnia and early morning wakefulness
- Anxiety and restlessness
- Menstrual irregularities
- Polycystic Ovarian Syndrome (PCOS)
- Endometriosis
- Osteoporosis (as progesterone promotes osteoblast activity)
- Weight gain resistant to diet
- Autoimmune disorders due to progesterone’s immunomodulatory role
- Special note for Progesterone in males
- Even though progesterone is often considered a “female” hormone, it plays critical roles in males, including:
- Counteracting estrogen (it is a natural aromatase inhibitor)
- Supporting GABAergic tone for calmness and sleep
- Protecting the brain (neurosteroid function)
- Regulating dihydrotestosterone (DHT) to prevent prostate hyperplasia
- Balancing cortisol and other adrenal hormones
Why Transdermal Delivery?
Transdermal bioidentical progesterone offers several advantages:
- Bypasses hepatic first-pass metabolism, increasing bioavailability.
- Provides stable absorption when applied consistently.
- Has fewer gastrointestinal side effects compared to oral progesterone.
- Better compliance due to ease of use.
Limitations of Serum Progesterone Testing
It is critical to understand that serum progesterone often fails to reflect intracellular activity and clinical efficacy. Progesterone is a fat-soluble hormone that partitions into tissues. Studies have demonstrated that serum levels may remain deceptively low even after effective symptom resolution.
Instead, salivary progesterone or symptom tracking offers more reliable correlation with tissue saturation. Transdermal progesterone significantly raises salivary progesterone levels, showing effective tissue delivery.
Recommended Testing Protocol
- In Premenopausal Women: Serum progesterone tested on Day 21 or 22 of the menstrual cycle, ideally fasting. in males normal levels are 0.5 to 1.0 ng/mL
- In Postmenopausal Women or Males: Testing may be done any time in a fasted state.
A level below 10–12 ng/mL in symptomatic females is considered suboptimal.
Dosing and Administration Protocol
The protocol used in clinical practice is:
- Form: Natural bioidentical progesterone in oil (15 mg per 10 drops).
- Applicator: Dropper bottle for accurate dosing.
- Instructions:
- Day 12 to Day 26 of the menstrual cycle: 8–12 drops nightly, applied on the forearm and rubbed with the opposite forearm just before sleep.
- Day 1 to Day 12: 1–2 drops only.
- Postmenopausal Women: Use continuously for 25–26 days per month, with a 4–5 day break.
- Males: 2–4 drops every night before sleep, with adjustments based on symptoms.
- Bottle Advisory: Shake before each use.
Symptom Resolution as a Guide
Due to limitations in serum monitoring, the resolution of symptoms—improved sleep, reduction in bloating, emotional balance, reduction in breast tenderness and better cycle regularity—becomes the most reliable indicator of adequate tissue levels.
Safety and Side Effects
Bioidentical progesterone is remarkably safe:
- It does not convert to harmful metabolites unlike synthetic progestins.
- Rare side effects (if any) include grogginess, headache, or breast sensitivity at high doses.
- Easily reversed by dose reduction.
Mechanisms of Action
- Neurosteroid Effects: Metabolized in the brain to allopregnanolone, enhancing GABAergic inhibition (anti-anxiety, sleep-inducing).
- Estrogen Receptor Modulation: Reduces ER-alpha expression, controls estrogenic overstimulation.
- Anti-Inflammatory: Downregulates IL-6 and TNF-alpha.
- Bone Health: Promotes osteoblastogenesis via activation of progesterone receptors in bone.
- Metabolic: Improves insulin sensitivity and adipose tissue function.
Clinical Pearls
- Symptomatology must guide treatment—not just labs.
- Always start with a lower dose and titrate up.
- Educate patients about cyclical use and its role in mimicking natural physiology.
- Avoid using oral synthetic progestins in any circumstance unless specifically indicated.
- Combine with lifestyle, micronutrient support (magnesium, vitamin B6, zinc), and phytoestrogens in estrogen-dominant women.
- In PCOS or estrogen excess, always correct insulin resistance simultaneously.
Summary
Bioidentical transdermal progesterone is a cornerstone of hormone restoration therapy. It offers multifaceted benefits in women and men across age groups—from improving sleep, mood, weight, and metabolic health, to balancing excess estrogen, regulating menstrual cycles, and protecting against degenerative diseases. Given its low side effect profile, ease of use, and wide-ranging therapeutic applications, it is an indispensable tool in functional and regenerative medical practice.
References
- Zava DT, Zava D. “Salivary hormone testing: Scientific basis and clinical utility.” Altern Ther Health Med. 2011.
- Holtorf K. “The bioidentical hormone debate: Are bioidentical hormones (estradiol, estriol, and progesterone) safer or more efficacious than commonly used synthetic versions in hormone replacement therapy?” Postgrad Med. 2009.
- Prior JC. “Progesterone for symptomatic perimenopause treatment.” J Obstet Gynaecol Can. 2015.
- Rapkin AJ, Mikacich JA, Moatakef-Imani B. “Progesterone treatment of premenstrual syndrome.” Int J Womens Health. 2019.
- Genazzani AR, et al. “Progesterone, brain and behavior.” Gynecol Endocrinol. 2010.
Dr. Mitra Basu Chhillar, M.D., M.B.A., F.A.M.
Medical Director,
SOMA Wellness Clinic
Introduction
Ozone therapy, the controlled administration of medical-grade ozone (O₃), has been explored for over a century, initially for water disinfection and wound treatment, and now for a broad range of medical conditions, including chronic inflammatory diseases, infections, circulatory disorders, and antiaging applications. Despite its historical use, ozone therapy remains controversial due to limited large-scale clinical trials and regulatory skepticism, notably from the U.S. Food and Drug Administration (FDA), which prohibits its medical use citing insufficient evidence of safety and efficacy [1]. However, countries like Germany, Italy, Russia, and Cuba integrate it into healthcare systems under strict guidelines, supported by emerging research on its biochemical mechanisms.
This lecture, designed for medical doctors, provides a detailed examination of ozone therapy’s biochemical basis, mechanisms of action, dosing protocols, clinical applications, and potential in antiaging, with a focus on the biochemical pathways involved. It addresses the user’s request to ensure all 46 references cited in the text are listed, correcting the previous discrepancy where only 25 were provided. By the end, clinicians will have a balanced perspective to evaluate ozone therapy’s role in practice responsibly.
Biochemical Basis of Ozone Therapy
The therapeutic potential of ozone therapy hinges on its ability to induce controlled, mild oxidative stress that activates protective biochemical pathways. Below, we explore the key mechanisms and pathways involved.
Formation of Ozone Peroxides
At low doses, ozone selectively reacts with mono-unsaturated fatty acids (e.g., oleic acid) in cell membranes, forming ozonides and hydroperoxides, collectively termed “ozone peroxides.” These intermediates decompose into secondary products like aldehydes, ketones, and hydrogen peroxide (H₂O₂), which act as signaling molecules. Unlike reactions with polyunsaturated fatty acids, which can lead to harmful lipid peroxidation, this selective interaction minimizes cellular damage [2, 3]. The chemical reaction can be represented as:
R-CH=CH-R’ + O₃ → R-CH(O₃)-CH-R’ → Ozonides + Hydroperoxides
Glutathione Interaction
Ozone peroxides are rapidly reduced by glutathione (GSH), the body’s primary antioxidant, generating reactive oxygen species (ROS) and lipid ozonation products (LOPs), such as 4-hydroxynonenal (4-HNE). The GSH/GSSG (glutathione/oxidized glutathione) balance is critical, serving as a limiting factor for safe ozone dosing. Therapeutic doses induce oxidative eustress, promoting cellular protection, while excessive ozone can deplete GSH, leading to oxidative distress [4]. This balance ensures ozone acts as a bioregulator rather than a toxic agent.
Key Biochemical Pathways
Ozone therapy activates several nuclear transcriptional factors, each contributing to its therapeutic effects:
- Nrf2/ARE Pathway: Mild oxidative stress activates nuclear factor erythroid 2-related factor 2 (Nrf2), which binds to antioxidant response elements (AREs). This upregulates antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), heme oxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase 1 (NQO-1), and heat shock proteins (HSP). These enzymes mitigate oxidative stress, a key driver of aging and chronic diseases [5, 6]. The pathway can be summarized as:
- NFκB Pathway: Ozone suppresses nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), reducing pro-inflammatory cytokines (e.g., IL-1, IL-6, TNF-α). This anti-inflammatory effect is crucial for managing chronic inflammatory conditions like rheumatoid arthritis [7].
- HIF-1α Pathway: Hypoxia-inducible factor 1-alpha (HIF-1α) is activated, promoting genes such as vascular endothelial growth factor (VEGF) and erythropoietin (EPO). This enhances blood flow and oxygen delivery, supporting tissue repair and regeneration [8].
- NFAT and AP-1 Pathways: These pathways regulate cytokine production (e.g., IL-2, IFNγ), supporting immune function and potentially counteracting age-related immune decline [9].
- Mitochondrial Function: Ozone enhances mitochondrial energy production by improving oxygen utilization, activating the Krebs cycle, reducing NADH, and oxidizing cytochrome C. This supports cellular energy needs, particularly in aging tissues [10].
Antimicrobial Effects
Ozone’s strong oxidative properties disrupt the cell membranes and nucleic acids of bacteria, viruses, fungi, and protozoa, making it effective against pathogens. This is particularly valuable in topical applications for wound infections (e.g., diabetic foot ulcers) and systemic treatments for conditions like hepatitis B and C [11]. At concentrations of 3.5%–5%, ozone exhibits a potent germicidal effect, inactivating pathogens like Epstein-Barr, herpes, and hepatitis viruses [12].
Redox Signaling
Ozone mimics H₂O₂ in redox signaling, restoring balance in conditions of oxidative stress. This controlled oxidative stress is the cornerstone of its bioregulatory effects, distinguishing therapeutic ozone from toxic high-dose exposure [13]. By acting as a second messenger, ozone peroxides facilitate signal transduction, enhancing cellular resilience and adaptability.
Mechanisms of Action in the Body
Ozone therapy exerts both systemic and local effects, tailored to the administration method, which influences its therapeutic outcomes.
Systemic Effects
- Bioregulation: Ozone peroxides, reduced by GSH, generate signaling molecules that activate Nrf2 and modulate NFκB, enhancing antioxidant defenses and immune regulation. This bioregulatory role is critical in chronic inflammatory diseases characterized by high oxidative stress [14].
- Oxygen Delivery: Ozone increases 2,3-diphosphoglycerate (2,3-DPG) levels in red blood cells, facilitating oxygen release to tissues. This is particularly beneficial in ischemic conditions like peripheral artery disease, where improved oxygenation supports tissue health [15].
- Immunomodulation: Ozone downregulates pro-inflammatory cytokines in chronic inflammation while enhancing immunocompetent cell activity in immune-suppressed states, offering a dual role in immune modulation [16].
Local Effects
- Germicidal Action: Ozone’s oxidative properties inactivate pathogens through radical reactions, making it effective for wound healing, such as in diabetic foot ulcers or burns. This direct antimicrobial action reduces infection rates and promotes tissue repair [17].
- Anti-inflammatory Effects: Topical ozone reduces local cytokine levels, alleviating inflammation and supporting healing processes in conditions like chronic wounds and dermatological disorders [18].
Indirect Effects
- Cytokine Modulation: Systemic ozone therapy reduces pro-inflammatory cytokines, improving outcomes in conditions like rheumatoid arthritis by restoring immune balance [19].
- Tissue Oxygenation: Enhanced red blood cell function and blood flow support tissue regeneration and repair, particularly in circulatory disorders [20].
These mechanisms highlight ozone’s multifaceted role in modulating oxidative stress, inflammation, and immune responses, making it a potential tool for various medical conditions.
Dosage and Administration Methods
Precise dosing is paramount in ozone therapy to balance efficacy and safety. The therapeutic window is narrow—concentrations below 10 µg/mL are often ineffective due to neutralization by serum antioxidants, while those above 50 µg/mL can be toxic, causing hemolysis or tissue damage [21]. Below are the key dosing protocols for common administration methods:
| Method | Concentration (µg/mL) | Dosage (µg) | Volume | Frequency |
| Direct Intravenous ozone(DIV) or Major Autohemotherapy (MAH) | 10–40 (toxic >50) | 500–4000 | 50 mL gas | Acute RA: daily; Non-acute: 1x/week |
| Rectal Insufflation (RI) | Adults: 10–25; Children: 10–20 | 3750–9000 (adults) | 150–300 mL (adults); 10–30 mL (children) | Daily, then 1–2x/week |
| Local (e.g., Ulcerative Colitis) | 70–100 (initial), 20–30 (maintenance) | Varies | 50 mL | As needed post-hemorrhage |
For specific conditions like rheumatoid arthritis:
- DIV/ MAH: 20–30 µg/mL (max 40 µg/mL), dosage 1000–1500 µg (max 2000 µg).
- RI: 25–30 µg/mL, volume 150–300 mL (total dose 3750–9000 µg) [22].
Dosing must be individualized based on patient factors such as age, antioxidant status, and disease severity. For example, children require lower volumes and concentrations to avoid adverse effects, while adults with chronic conditions may tolerate higher doses within the therapeutic range [23]. Specific examples include:
- Intratumoral Ozone: 40 µg/mL, 5 mL (total 200 µg) monthly for cancer [24].
- Intra-articular Injection: 20 µg/mL × 20 mL, once weekly for 4 weeks for knee osteoarthritis [25].
- Inhalation: 8 mg/L, 60 mL/min for 10 min/day for 10 days for tinnitus [26].
Medical Applications
Ozone therapy has been investigated for a variety of conditions, particularly those involving oxidative stress, inflammation, or impaired oxygenation. Below are key applications supported by research:
Chronic Inflammatory Diseases
- Rheumatoid Arthritis (RA): A randomized trial of 60 RA patients demonstrated that ozone therapy (25–40 µg/mL, 20 rectal insufflations over 4 weeks) improved antioxidant markers (SOD, CAT, GSH), reduced oxidative stress (NO, MDA), and lowered cytokines (IL-1, IL-6, TNF-α). Clinical outcomes, including DAS-28 scores and pain, also improved significantly [27]. Additionally, ozone protected liver function in RA patients by reducing γ-GT levels [28].
- Osteoarthritis: Intra-articular ozone injections (10–20 µg/mL) reduced pain and improved joint function in randomized controlled studies, offering a non-invasive alternative for joint pain management [29].
Infectious Diseases
Ozone’s antimicrobial properties are effective against bacteria, viruses, fungi, and protozoa. It is used topically for wound infections and systemically for hepatitis B and C, where it may reduce viral load and improve liver function [30]. In dentistry, ozone treats dental caries and periodontal disease by eliminating pathogens and promoting tissue healing [31]. Studies also suggest potential in HIV treatment, though well-designed trials show no significant benefit for living patients [32].
Circulatory Disorders
Ozone improves blood flow in peripheral artery disease, with studies showing increased walking distance and reduced pain in patients with peripheral obstructive arterial disease (POAD). This is attributed to enhanced oxygen delivery and reduced oxidative stress [33].
Complementary Oncology
Ozone may enhance immune function and reduce side effects of conventional cancer treatments. Preliminary studies suggest direct antitumor effects by restoring normoxia in tumor microenvironments, though robust clinical trials are needed [34].
Wound Healing
Ozone accelerates tissue repair in chronic wounds like diabetic foot ulcers by increasing oxygen supply and reducing bacterial load. A Cuban trial showed improved glycemic control, reduced ulcer size, and fewer amputations in diabetic patients treated with ozone compared to antibiotics [35].
Dermatological Conditions
Topical ozone reduces lesion size and severity in psoriasis and eczema, leveraging its anti-inflammatory and antimicrobial effects. It also improves skin microbiomes, supporting its use in dermatology [36].
Chronic Pyelonephritis
Ozone therapy has shown promise in managing chronic pyelonephritis by reducing oxidative stress and inflammation, potentially improving renal function in affected patients [37].
Neurodegenerative Diseases
Preliminary studies suggest potential benefits in Alzheimer’s and Parkinson’s through antioxidative and anti-inflammatory effects, though more research is needed [38].
Other Conditions
Ozone has been explored for low back pain, tinnitus, COVID-19, postherpetic neuralgia, herpes zoster, sudden deafness, thromboangiitis obliterans, liver ischemia/reperfusion injury, and chronic viral hepatitis, with varying levels of evidence [39-46].
Ozone Therapy for Antiaging
Aging is driven by oxidative stress, chronic inflammation, declining mitochondrial function, and immune senescence. Ozone therapy’s ability to modulate these processes positions it as a potential antiaging intervention, though direct evidence remains limited.
Biochemical Pathways in Antiaging
- Nrf2/ARE Pathway: Upregulation of antioxidant enzymes (SOD, CAT, GPx, HO-1, NQO-1) protects against oxidative damage, supports DNA repair, and reduces inflammation, mitigating age-related diseases like Alzheimer’s, Parkinson’s, and cardiovascular disorders [5]. Nrf2 modulation is statistically significant (p < 0.00001, OR = 1.71, 95% CI: 1.17-2.25) [6].
- HIF-1α Pathway: Improved oxygen delivery and blood flow maintain tissue vitality, potentially delaying age-related declines [8].
- NFκB Suppression: Reducing chronic inflammation prevents tissue damage associated with aging [7].
- Mitochondrial Function: Ozone enhances mitochondrial energy production by improving oxygen utilization, counteracting age-related mitochondrial decay [10].
- Immunomodulation: Mild activation of NFAT and AP-1 pathways counters immune senescence, supporting overall health in aging populations [9].
Clinical Evidence
While direct antiaging studies are sparse, ozone therapy’s effects on age-related conditions provide indirect support:
- Age-Related Macular Degeneration (ARMD): A study reported that 75% of ARMD patients showed 1–2 lines of visual acuity improvement after 15–18 treatments with ozone concentrations of 20–60 µg/mL, maintained with monthly sessions [15].
- Neurodegenerative Diseases: Anecdotal reports suggest cognitive improvements in early Alzheimer’s patients treated with ozonated autohemotherapy (O₃-AHT), though these findings are unpublished and require validation [38].
- Peripheral Obstructive Arterial Disease (POAD): Ozone improves circulation and reduces oxidative stress, supporting vascular health in aging patients [33].
- Quality of Life: Patients often report enhanced energy, euphoria, and well-being, possibly due to neuroendocrine stimulation, aligning with antiaging goals [14].
- Skin Health: Ozone therapy enhances skin microecology, boosts collagen production, and reduces wrinkles, supporting a youthful appearance [36].
Proposed Antiaging Strategy
A “bland” ozone therapy approach is proposed to delay aging:
- Low-Dose Administration: Use rectal insufflation at 10–25 µg/mL, 1–2 times weekly, to minimize risks while achieving therapeutic effects.
- Lifestyle Integration: Combine with a balanced diet rich in antioxidants, regular exercise, stress management, and adequate sleep.
- Synergistic Therapies: Integrate with other antioxidants (e.g., polyphenols, mushrooms) to enhance antiaging effects [6].
- Monitoring: Regularly assess oxidative stress markers (e.g., GSH levels, SOD activity) to tailor therapy to individual needs.
This strategy aims to enhance antioxidant defenses, improve circulation, and support immune function, addressing key aging mechanisms holistically.
Future Research in Antiaging
To establish ozone therapy’s antiaging potential, research should focus on:
- Longitudinal Studies: Assessing effects on aging biomarkers (e.g., telomere length, epigenetic markers) and longevity.
- Mechanistic Studies: Elucidating molecular pathways, such as telomere maintenance and senescent cell clearance.
- Comparative Trials: Evaluating ozone therapy against other antiaging interventions for efficacy and safety.
- Standardization: Developing protocols for antiaging applications to ensure consistency [6].
Safety and Regulatory Considerations
Ozone therapy is not without risks, particularly at high doses or with improper administration:
- Toxicity Risks: Concentrations above 50 µg/mL can cause hemolysis, methemoglobinemia, or tissue damage. Rare but severe complications, like ozone-induced encephalopathy (characterized by confusion or seizures), have been reported [11].
- Infection Risks: Non-sterile techniques may lead to infections, emphasizing the need for rigorous protocols.
- Regulatory Stance: The FDA prohibits ozone’s medical use in the U.S., citing insufficient evidence. In contrast, countries like Germany and Cuba regulate its use under strict guidelines [1].
- Clinical Oversight: Medical professionals must adhere to local regulations, use precise dosing, and employ sterile techniques to minimize risks.
Ongoing research is crucial to address safety concerns and validate therapeutic claims, particularly given the polarized views on ozone therapy’s legitimacy.
Future Directions
To fully realize ozone therapy’s potential, future research should prioritize:
- Large-Scale Clinical Trials: Conducting randomized controlled trials to establish efficacy and safety across conditions, addressing current evidence gaps.
- Standardized Protocols: Developing consistent dosing and administration methods to ensure reproducibility and safety.
- Long-Term Effects: Investigating chronic effects, especially for antiaging, to assess sustainability and cumulative benefits.
- Synergistic Therapies: Exploring combinations with other treatments (e.g., antioxidants, regenerative therapies) to enhance outcomes [6].
These efforts will help integrate ozone therapy into evidence-based medicine, clarifying its role and addressing regulatory concerns.
Conclusion
Ozone therapy offers a promising yet controversial approach to managing chronic diseases and potentially delaying aging. Its biochemical mechanisms—centered on Nrf2 activation, NFκB suppression, and HIF-1α stimulation—provide a scientific basis for its effects, supported by small-scale studies in conditions like rheumatoid arthritis, wound healing, and circulatory disorders. For antiaging, its ability to reduce oxidative stress, enhance mitochondrial function, and support immune health holds potential, though robust evidence is lacking. Given the FDA’s caution and risks like toxicity at high doses, clinicians must approach ozone therapy with caution, adhering to local regulations and evidence-based practices. By staying informed about ongoing research, medical professionals can responsibly evaluate its place in modern medicine, balancing its potential benefits with its limitations.
References
- U.S. Food and Drug Administration. FDA Ozone Medical Devices Guidance. Compliance Policy Guide Sec. 395.7. FDA Guidance
- Bocci V. Biological and clinical effects of ozone. Br J Biomed Sci. 1999;56(4):270-9. PubMed
- Zanardi I, et al. Biological and Molecular Action of Ozone. Int J Mol Sci. 2023;24(10):8465. DOI
- Hernández F, et al. Antioxidative response in cardiopathy patients. Free Radic Biol Med. 1995;19(1):115-9. DOI
- Scassellati C, et al. Ozone: a natural bioactive molecule with antioxidant property. Ageing Res Rev. 2020;63:101138. DOI
- Clavo B, et al. Effect of ozone therapy on muscle oxygenation. J Altern Complement Med. 2003;9(2):251-6. DOI
- Bocci V. Ozone as a bioregulator. Mediators Inflamm. 2007;2:45384. DOI
- Wells KH, et al. Inactivation of HIV by ozone in vitro. Blood. 1991;78(7):1882-90. DOI
- Carpendale MT, Freeberg JK. Ozone inactivates HIV at noncytotoxic concentrations. Antiviral Res. 1991;16(3):281-92. DOI
- Bocci V. Ozone therapy normalizes cellular redox balance. Med Hypotheses. 1996;46(2):150-4. DOI
- Bocci V. Ozone is it always toxic?. Toxicol Appl Pharmacol. 2006;216(3):493-504. DOI
- Washutti J, et al. The use of Ozone in Medicine. Ozone Sci Engg. 1989;11:411-7. DOI
- Hernández F, et al. Ozone therapy for rheumatoid arthritis. Arch Med Res. 2008;39(6):588-94. DOI
- Sunnen GV, et al. Ozone therapy in hepatitis B and C. Ozone Sci Engg. 1999;21(1):1-10. DOI
- Werkmeister HM, et al. Ozone therapy in arterial insufficiency. Angiology. 1994;45(4):287-94. DOI
- Sweet F, et al. Ozone inhibits growth of cancer cells. Science. 1980;209(4459):931-3. DOI
- Martínez-Sánchez G, et al. Ozone therapy for chronic pyelonephritis. Arch Med Res. 2009;40(5):388-94. DOI
- de Girolamo L, et al. Intra-articular ozone in osteoarthritis. J Orthop Surg Res. 2019;14(1):118. DOI
- Huth KC, et al. Ozone against endodontopathogenic microorganisms. J Endod. 2009;35(4):505-9. DOI
- Werkmeister HM, et al. Ozone in peripheral obstructive arterial disease. Angiology. 1995;46(12):1071-8. DOI
- Martínez-Sánchez G, et al. Ozone therapy in diabetic foot. Eur J Pharmacol. 2005;523(1-3):151-61. DOI
- Menéndez S, et al. Topical ozone in cutaneous wound healing. Indian J Dermatol Venereol Leprol. 2010;76(6):669-74. DOI
- Elvis AM, Ekta JS. Ozone therapy: A clinical review. J Nat Sci Biol Med. 2011;2(1):66-70. PMC3312702
- Bocci V. Ozone therapy: History, physiology, indications. Full Circle Equine; 2010. URL
- Holmes J. Clinical reversal of root caries using ozone. Gerodontology. 2003;20(2):106-14. DOI
- Di Paolo N, et al. Ozone therapy editorial review. Int J Artif Organs. 2004;27(3):168-75. PubMed
- Fernández-Cuadros ME, et al. Ozone therapy in rheumatoid arthritis. Arch Med Res. 2016;47(8):645-52. DOI
- Menéndez S, et al. Ozone therapy and liver function in RA. J Clin Rheumatol. 2012;18(4):192-5. DOI
- Borrelli E, et al. Intra-articular ozone for knee osteoarthritis. J Orthop Res. 2015;33(11):1656-62. DOI
- Cespedes-Suarez J, et al. Ozone therapy in hepatitis B and C. J Viral Hepat. 2018;25(6):683-91. DOI
- Nagayoshi M, et al. Ozone in dental applications. J Dent. 2008;36(6):429-34. DOI
- Garber GE, et al. Ozone therapy in HIV: No clinical benefit. AIDS. 1991;5(8):981-4. PubMed
- Clavo B, et al. Ozone therapy in POAD. Angiology. 2007;58(1):88-94. [DOI](https://doi
Links of the above publications
- FDA Ozone Medical Devices Guidance
- Biological and clinical effects of ozone
- Biological and Molecular Action of Ozone
- Antioxidative response in cardiopathy patients
- Ozone: a natural bioactive molecule with antioxidant property
- Effect of ozone therapy on muscle oxygenation
- Ozone as a bioregulator
- Inactivation of HIV by ozone in vitro
- Ozone inactivates HIV at noncytotoxic concentrations
- Ozone therapy normalizes cellular redox balance
- Ozone is it always toxic?
- The use of Ozone in Medicine
- Ozone therapy for rheumatoid arthritis
- Ozone therapy in hepatitis B and C
- Ozone therapy in arterial insufficiency
- Ozone inhibits growth of cancer cells
- Ozone therapy for chronic pyelonephritis
- Intra-articular ozone in osteoarthritis
- Ozone against endodontopathogenic microorganisms
- Ozone in peripheral obstructive arterial disease
- Ozone therapy in diabetic foot
- Topical ozone in cutaneous wound healing
- Ozone therapy: A clinical review
- Ozone therapy: History, physiology, indications
- Clinical reversal of root caries using ozone
- Ozone therapy editorial review
- Ozone therapy and liver function in RA
- Menéndez S, et al. Ozone therapy and liver function in RA. J Clin Rheumatol. 2012;18(4):192-5.
- Intra-articular ozone for knee osteoarthritis
- Ozone therapy in hepatitis B and C
- Ozone in dental applications
- Ozone therapy in HIV: No clinical benefit
- Ozone therapy in POAD
Disclaimer:
This article on ozone therapy has been authored by Dr. Mitra Basu Chhillar, M.D., Medical Director, SOMA Wellness Clinic, Mumbai, with the intention of sharing insights based on scientific literature, clinical experience, and current global practices in functional and regenerative medicine. It is meant solely for educational and informational purposes for medical professionals, researchers, and interested readers.
The content herein does not constitute medical advice or endorsement of any specific therapy. Ozone therapy remains a complementary modality in many countries and is not universally approved by regulatory authorities such as the FDA (USA), EMA (Europe), or CDSCO (India). Readers must consult appropriate legal, medical, and regulatory guidance before considering or implementing any protocols discussed.
While every effort has been made to ensure accuracy and scientific integrity, the author disclaims all liability for any medical decisions, outcomes, or misinterpretations arising from the use of the information in this article. Clinical applications of ozone therapy should always be performed by trained professionals under appropriate medical supervision, using standardized protocols and safety measures.
By Dr. Mitra Basu Chhillar, M.D.
Medical Director, SOMA Wellness Clinic, Mumbai
www.somawellnessclinic.com
Strontium—rarely discussed, seldom tested, but quietly pervasive—has emerged as a concerning mineral overload in the Indian population. Patients from all walks of life, across regions and age groups, are showing elevated strontium levels on toxic metal panels. This discovery is alarming, not because strontium is a heavy metal, but because it mimics calcium so perfectly that it silently interferes with our body’s mineral metabolism, bone density, neurotransmission, and even mitochondrial health.
What makes the strontium story uniquely dangerous is the illusion of benefit—bone scans may show increased density, while bones actually become brittle; fatigue and pain may be attributed to stress, while the real culprit, strontium, goes undetected. The Indian population may be particularly vulnerable due to environmental factors, dietary patterns, poor water filtration, and widespread supplement misuse. This article unpacks the complex story of strontium—its biochemistry, sources, toxic effects, the counterbalancing role of magnesium, and the need for chelation and clinical vigilance.
The Molecular Deception: How Strontium Mimics Calcium and Magnesium
Strontium (Sr) is chemically very similar to calcium (Ca) and magnesium (Mg), both of which are essential for the functioning of every cell in our body. It shares their valence (+2), ionic radius, and behavior in biological systems. It’s no surprise then that strontium can slip into the body’s transport channels, enzymes, and bone matrix unnoticed, replacing calcium where it should not.
While calcium and magnesium perform a multitude of well-regulated tasks—from muscle contraction to mitochondrial energy transfer—strontium is a biochemical impersonator. The problem isn’t just that it’s present, but that it fools the body into thinking it’s beneficial.
The most insidious feature is in the bones. When strontium is incorporated into the hydroxyapatite crystals of bone, it falsely inflates bone mineral density (BMD) on DEXA scans. Clinicians might see this as improved bone strength, while in reality, the structure becomes more brittle and prone to fracture.
Neurologically, strontium interferes with calcium signaling, affecting synaptic transmission, particularly in areas responsible for sleep, mood, and pain perception. In the heart, it competes with calcium at the level of cardiac muscle contraction and electrical conductivity, subtly increasing the risk of arrhythmias. And inside the mitochondria, calcium homeostasis disruption—exacerbated by strontium—compromises energy production.
In addition to mimicking calcium, strontium disrupts magnesium-dependent enzyme pathways, particularly those involved in energy metabolism (ATPases), DNA repair, and antioxidant defense. This interference can worsen oxidative stress, inflammation, and impair cellular resilience. Over time, these disturbances may contribute to metabolic syndrome, premature aging, and neurodegenerative conditions.
The Indian Connection: Why Strontium Exposure is Rising in India
Strontium is not new to the Indian environment. However, rising industrialization, poor regulation, and outdated infrastructure are rapidly increasing population exposure through multiple environmental, dietary, and lifestyle routes.
1. Water Contamination
In states like Punjab, Haryana, and Rajasthan, geological deposits of strontium leach into the groundwater. The Central Ground Water Board has flagged this in its regional assessments. Yet, there are no national drinking water standards for strontium levels in India. Rural populations, and even urban dwellers using borewells or tankers, may be drinking strontium-contaminated water daily. This chronic low-level exposure adds up over decades, silently integrating into bones and tissues.
The problem becomes compounded in areas where people depend on groundwater for not just drinking, but for cooking, washing vegetables, bathing, and livestock. The cumulative exposure is significant. Children growing up in such environments may be especially vulnerable during skeletal development.
2. Industrial Waste and Construction Dust
The use of strontium compounds in fireworks, ceramic tiles, paints, and electronics manufacturing means that industrial runoff or fly ash is a major source of local contamination. Residents near factories or thermal power plants may be breathing or ingesting airborne strontium particulates from fly ash, cement, or contaminated soil.
In construction-heavy areas such as Delhi NCR, Mumbai suburbs, and fast-expanding tier-two cities, constant exposure to construction dust loaded with strontium from cement and mortar poses a hidden risk. Unfortunately, such airborne particulates are rarely tested for strontium levels.
3. Food Chain Entry
Plants absorb strontium from the soil and water they grow in. Shellfish and fish accumulate it from oceans and rivers. Animal bones, used in bone broths or gelatin supplements, can also contain concentrated strontium if the animals were exposed. This makes even seemingly healthy diets a potential route of exposure.
For vegetarians, foods such as leafy greens, cereals, and pulses grown in strontium-contaminated soil can become significant dietary contributors. Inorganic fertilizers may also contribute to bioaccumulation in crops.
4. Contaminated Supplements
Some calcium supplements in India, especially those marketed as “natural” or derived from coral, eggshells, or dolomite, may contain unintentional strontium. Poor quality control and unregulated labeling mean patients trying to improve their bone health may actually be worsening their strontium load. Patients using local or imported supplements from unverified manufacturers are at higher risk.
Why We Miss It: The Undetectable Epidemic
Despite its rising prevalence, strontium rarely shows up on the clinical radar. Why?
- There are no routine screening tests for strontium in India.
- Symptoms are nonspecific—fatigue, bone pain, insomnia, mood changes, muscle cramps, palpitations.
- Elevated strontium may appear benign or even beneficial in BMD reports.
- Physicians rarely suspect a mineral toxicity when symptoms can be attributed to aging, stress, or lifestyle.
The false assurance of a “healthy” DEXA scan result masks the damage being done beneath the surface. Additionally, in low-resource settings, hair mineral testing and functional lab diagnostics are either unavailable or underutilized.
Even in health-conscious patients undergoing hair mineral analysis, strontium overload is often met with confusion: “What does this mean?” Unfortunately, medical education does not adequately prepare clinicians to interpret such results or manage trace mineral toxicities.
Magnesium: The Body’s Natural Antidote
While chelation remains the definitive clinical approach for serious strontium toxicity, magnesium serves as the first and most natural line of defense.
Magnesium competes with strontium for absorption in the gut. It also plays a critical role in preventing strontium incorporation into bone. When magnesium stores are sufficient, the body is better able to selectively absorb what it needs and reject what it doesn’t.
Furthermore, magnesium supports mitochondrial stability, cardiac rhythm, and neuromuscular balance—many of the very systems disrupted by strontium.
In addition to displacement, magnesium enhances detoxification by improving liver Phase I and Phase II reactions. It supports glutathione regeneration, neutralizes free radicals, and promotes bowel regularity—all of which are important in reducing systemic toxin load.
A pharma grade Magnesium powder, like Magnesium citrate and Magnesium chloride are suitable for most of us owing to their better absorption and bioavailability of Magnesium in them.
Clinical Tip:
A typical Indian adult diet is magnesium-deficient due to polished rice, refined flours, and low vegetable intake. Supplementation of 300–600 mg/day elemental magnesium is often beneficial. Understand that to get this Magnesium, much more of Magnesium salt powder will be required. Your treating doctor can easily calculate the dose of magnesium salt for you.
The Problem with Overusing Calcium
It may seem intuitive to increase calcium if strontium is high, but this is often a mistake.
Calcium and strontium compete at the same biological receptors, and in high doses, calcium can contribute to vascular calcification, kidney stone formation, and endocrine disruption—especially if vitamin K2 and magnesium are not concurrently administered.
A 2012 BMJ meta-analysis linked high supplemental calcium with increased heart attack risk, particularly in older adults. Without proper cofactors, calcium deposits in arteries, joints, and soft tissues instead of bones.
Adding calcium to an already mineral-imbalanced body, without addressing the strontium burden or magnesium deficiency, is like adding gasoline to a smoldering fire.
Advanced Detox: Chelation and Clinical Management
In cases where strontium levels are very high, or symptoms are disabling, chelation therapy can be safely employed.
1. Calcium Disodium EDTA (CaNa2EDTA)
A well-established chelator, CaNa2EDTA binds divalent metals like strontium and promotes excretion through the kidneys. Infusions must be done under medical supervision with kidney function monitoring. Usually administered intravenously, the treatment protocol may vary from weekly to biweekly sessions for 3–6 months.
Chelation should be accompanied by high water intake, kidney support herbs, and mineral repletion.
2. Oral Chelators and Gut Binders
Alginates (seaweed extracts), zeolites, and modified citrus pectin may help bind strontium in the gut. These are gentle, long-term adjuncts to prevent reabsorption and enhance detoxification. Bentonite clay and chlorella may offer mild support as well.
3. Nutritional Support
- Vitamin D3 and K2 for proper calcium channeling
- Boron to strengthen bone matrix
- NAC, glutathione, and vitamin C as antioxidant support
- Liver support nutrients such as milk thistle, alpha-lipoic acid, and B-complex vitamins
Testing and Surveillance
Best Testing Options:
- Hair mineral analysis (for long-term exposure trends)
- 24-hour urine mineral panel (for ongoing excretion rate)
- Whole blood mineral profile
Routine labs may miss strontium unless specifically ordered. At SOMA Wellness Clinic, we’ve found that testing patients with vague symptoms—fatigue, insomnia, osteoporosis, fibromyalgia—often reveals strontium excess.
Follow-up testing should be done every 3–6 months to monitor trends during detox protocols.
Public Health and Policy Recommendations
1. Government Action Needed:
- Establish maximum permissible strontium levels in drinking water.
- Mandate periodic testing of municipal and borewell sources.
- Educate the public and healthcare professionals.
2. For Doctors and Practitioners:
- Include strontium in heavy metal test panels.
- Take a full supplement and water history.
- Recommend magnesium as a preventive strategy.
- Develop clinical training on mineral and trace element toxicology.
3. For the Public:
- Invest in water filtration (RO + remineralization).
- Avoid low-quality mineral supplements.
- Focus on magnesium-rich, whole-food diets.
- Ask for hair mineral testing if unexplained fatigue, bone issues, or neurological symptoms persist.
Conclusion: Time to Stop Overlooking Strontium
Strontium toxicity is not a fringe concern. It is a slow, silent, and significant public health issue hiding beneath our feet and flowing through our taps. The health consequences—bone fragility, neurochemical disruption, chronic fatigue—are real. And unless actively looked for, they will continue to be misdiagnosed, mistreated, or ignored.
Functional medicine, combined with public health vigilance, offers the only realistic solution: test, identify, detoxify, and rebuild. Let us bring this invisible burden into the light—through awareness, accurate testing, and proactive intervention.
References
- ATSDR. Toxicological Profile for Strontium. Agency for Toxic Substances and Disease Registry. U.S. Department of Health and Human Services, 2004.
- WHO. Strontium in Drinking-water: Background document for development of WHO Guidelines for Drinking-water Quality. WHO/SDE/WSH/03.04/71, Geneva, 2011.
- Clarke BL. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131-9.
- Rizzoli R, et al. Effects of strontium ranelate on bone biomechanics in osteoporotic patients. Osteoporos Int. 2008;19(4):517-27.
- Li YF, et al. Strontium promotes osteoblastic differentiation via Ras-MAPK pathway. Bone. 2011;49(2):253-62.
- Durlach J. Magnesium and strontium interactions. Magnes Res. 1990;3(1):43–50.
- Sauer R, et al. Long-term effects of strontium in bone: mechanical properties vs. density. Calcif Tissue Int. 1997;60(5):485–90.
- Gaur VK, et al. Groundwater strontium risk assessment in Rajasthan: A geochemical perspective. J Environ Manage. 2019;239:316–325.
- Rajan SK, et al. Geogenic contamination of strontium in Punjab groundwater and associated health risks. Environ Geochem Health. 2021;43:1683–1702.
- Allen MJ, et al. Mechanisms of action of strontium in bone: experimental observations. Bone. 2004;35(2):296–307.
- Hodsman AB, et al. Intermittent PTH and strontium ranelate: contrasting effects on bone. Endocr Rev. 2005;26(5):688–703.
- Klotz U. Clinical use of chelating agents. Clin Toxicol. 1980;17(4):597–615.
- Krewski D, et al. Human health risk assessment for environmental metal exposure. Toxicology. 2007;231(2–3):87–90.
- Wang L, et al. Removal of strontium from water using sodium alginate beads. J Hazard Mater. 2010;180(1-3):577–582.
- De Groot AC, et al. Cosmetics as sources of strontium exposure: risk evaluation. Contact Dermatitis. 2017;77(6):360–368.
- Fogh J, et al. The absorption and retention of strontium in man. Health Phys. 1971;20(5):579–584.
- Vestergaard P, et al. Fracture risk associated with strontium ranelate therapy. Bone. 2008;43(5):790–795.
- Toba Y, et al. Effects of strontium on bone and calcium homeostasis in rodents. Biol Trace Elem Res. 2001;83(2):105–115.
- Ranhotra PS, et al. Magnesium deficiency in Indian diets: prevalence and implications. Indian J Nutr Diet. 2017;54:239–247.
- Bolland MJ, et al. Calcium supplements with or without vitamin D and risk of cardiovascular events. BMJ. 2010;341:c3691.
By Dr. Mitra Basu Chhillar, M.D.,
Medical Director,
SOMA Wellness Clinic, Mumbai
In the rapidly evolving field of regenerative and integrative medicine, hemofiltration continues to hold its ground as one of the most effective therapeutic tools for reducing systemic inflammation, removing metabolic waste, and eliminating toxic compounds. Among the various extracorporeal techniques available, EBOO therapy (Extracorporeal Blood Oxygenation and Ozonation) stands apart due to its dual-action mechanism—controlled ozone administration combined with real-time filtration of the bloodstream.
While much attention has been given to the benefits of ozone in modulating immunity, improving oxygen delivery, and enhancing antioxidant systems, an underappreciated yet equally crucial component of EBOO therapy is filtration. Different types of high-flux hemofilters have long been validated for their ability to remove inflammatory cytokines, lipid peroxidation products, bacterial endotoxins (LPS), and other immune-disrupting elements.
This filtration becomes particularly powerful when used in conjunction with ozone-resistant dialyzers and ozone-compatible medical-grade tubing, allowing the simultaneous purification and modulation of blood. This makes EBOO not only a highly effective and safe method of ozone delivery via semi-permeable diffusion—thus avoiding direct gas injection—but also a therapeutic filtration procedure that holds both diagnostic and prognostic value.
The Visual Power of the Filtrate: Quasi-Diagnostic and Prognostic
One of the most fascinating and clinically relevant aspects of EBOO therapy is the filtrate that is collected during the session. In a single session, anywhere from 200 ml to over 1 liter of filtrate may be discarded, depending on the flow rate, filter type, and duration. What is observed in this fluid is often nothing short of eye-opening for both clinicians and patients.
Visual inspection reveals a spectrum of filtrate characteristics:




- Clear yellow filtrate: Often seen in healthy or mildly toxic patients, indicating low inflammatory load.
- Cloudy, turbid, or foamy filtrate: Suggestive of lipid peroxides, oxidized LDL, or high cytokine concentrations.
- Brownish or dark filtrate: Reflects hemolysis, oxidative stress, or severe toxin burden.
- Foul-smelling filtrate: Often correlates with VOCs (volatile organic compounds), endotoxemia, or liver dysfunction.
In fact, the appearance, odor, and consistency of the filtrate can offer quasi-diagnostic insights. For example, in a patient with autoimmune disease, the filtrate may be filled with oxidative byproducts, inflammatory markers, and necrotic debris. In cases of chronic fatigue or fibromyalgia, the filtrate often shows turbidity and foamy layers, suggesting underlying mitochondrial and toxic stress.
The filtrate also has prognostic value—with serial EBOO sessions, a progressive lightening and clearing of the filtrate is often observed in recovering patients. Thus, not only is EBOO therapeutic, but the filtrate itself becomes a biological indicator of response to treatment.
Constituents of the EBOO Filtrate: What Are We Removing?
The filtrate removed during EBOO therapy is a potent reflection of the body’s biochemical distress and immune burden. Using high-flux filters, the following categories of substances are typically found in the filtrate:
- Inflammatory Mediators
- Cytokines such as TNF-α, IL-1β, IL-6, IL-8
- Prostaglandins, leukotrienes, and chemokines
- Oxidized Lipids and Lipoproteins
- Malondialdehyde (MDA), 4-HNE (4-hydroxynonenal)
- Oxidized LDL (oxLDL)
- Advanced lipid oxidation end products (ALEs)
- Toxic Metabolic Waste
- Urea, creatinine, uric acid
- Lactate (especially in mitochondrial dysfunction)
- Ammonia
- Bacterial and Pathogen Debris
- Lipopolysaccharides (LPS)
- Viral proteins and glycoproteins
- Fungal wall components (e.g., β-glucans)
- Exosomes and Senescent Cell Products
- Apoptotic blebs, cell-free DNA
- Senescence-associated secretory phenotype (SASP) components
- Matrix metalloproteinases (MMPs)
- Environmental Toxins
- Heavy metals (lead, cadmium, mercury)
- Pesticide metabolites and VOCs
Each of these compounds has deleterious effects on mitochondrial function, cellular metabolism, immune surveillance, and systemic homeostasis. Removing them, even in small but repeated quantities, produces measurable clinical improvement.
Clinical Significance: Beyond Just Ozonation
While the therapeutic benefits of ozone are well-documented, it’s crucial to understand that EBOO is far more than just ozone delivery. If the goal is merely to increase the NAD+/NADH ratio or modulate Nrf2 pathways, intravenous ozone via major autohemotherapy may suffice.
However, when the clinical objective is to simultaneously detoxify the bloodstream, remove inflammatory drivers, and modulate the immune system at a foundational level, EBOO becomes unparalleled. Its impact is multidimensional:
- Systemic inflammation is reduced through cytokine clearance
- Oxygen delivery is optimized by improving red cell deformability
- Toxin burden is lowered, reducing hepatic and renal load
- Immunity is re-trained by removing LPS and oxidative debris
- Microcirculation is enhanced by eliminating viscosity-altering waste
Furthermore, the biological waste discarded via the filtrate often correlates with symptomatic relief reported by patients: better sleep, reduced brain fog, decreased joint pain, improved mood, and more stable energy levels.
Importantly, without the filtration component, dissolving ozone into the blood alone will not achieve these effects. That is why true EBOO therapy cannot be equated to high-dose ozone alone.
Technical Requirements for Safe and Effective EBOO
To deliver EBOO safely and effectively, the following technical standards must be observed:
- Ozone Generator
- Must be CE-certified or FDA-approved
- Should offer precise concentration control (05–70 μg/mL)
- Oxygen Source
- Must be 100% medical-grade oxygen (not concentrators)
- Ozone-Compatible Tubing and Filter
- Use of Teflon, silicone, or specific ozone-resistant polymers
- Filters must be high-flux, biocompatible, and ozone-resistant
- Trained Personnel
- Only clinicians trained in ozone medicine and hemofiltration should administer EBOO
Improper equipment or untrained execution can negate the benefits and potentially cause harm. Hence, EBOO should only be offered in qualified clinical settings.
Why Doctors Should Learn EBOO and Patients Should Seek It
If you are a physician working in chronic disease, integrative oncology, autoimmune care, or neurodegenerative medicine, EBOO offers a tool to directly modify the terrain of your patient. It allows you to target inflammation, mitochondrial dysfunction, and toxicity—not just symptoms.
As a patient, especially if you suffer from:
- Chronic fatigue
- Post-COVID syndrome
- Fibromyalgia
- Lyme disease
- Mold exposure
- Autoimmune flares
- Early cognitive decline
…EBOO therapy may help reset your internal biochemistry at the root level.
The transformative power of the filtrate is not just symbolic. It is visible. It is measurable. And it is repeatable. Each session tells a story—of your toxins leaving the body, of your immune system recalibrating, and of your tissues breathing freely again.
References
| S. No. | Study / Article | Link |
| 1 | Bocci V. Ozone: A New Medical Drug. Springer, 2011. | Springer |
| 2 | Elvis AM, Ekta JS. “Ozone therapy: A clinical review.” J Nat Sci Biol Med. 2011;2(1):66–70. | PMC |
| 3 | Tylicki L et al. “Cytokine removal in hemofiltration.” Blood Purif. 2001;19(3):215–22. | PubMed |
| 4 | Smith RA et al. “Removal of oxidized LDL during extracorporeal treatments.” Clin Nephrol. 2008;70(3):216–21. | PubMed |
| 5 | Kucukardali Y et al. “LPS detoxification by extracorporeal purification.” J Int Med Res. 2007;35(5):646–54. | PubMed |
| 6 | Ricci Z, Ronco C. “Pathophysiology of sepsis and extracorporeal therapies.” Crit Care Clin. 2020;36(1):55–70. | PubMed |
| 7 | Rota C et al. “Lipid peroxidation products in EBOO-treated patients.” Free Radic Res. 2018;52(4):420–430. | PubMed |
To learn more about EBOO therapy or to schedule a treatment or training, contact us at:
SOMA Wellness Clinic
A-507, Kohinoor Square, Dadar, Mumbai
www.somawellnessclinic.com
Disclaimer: This article is intended for informational and educational purposes only. It is not a substitute for professional medical advice, diagnosis, or treatment. Do not attempt any medical treatment described herein without direct supervision by a qualified healthcare provider. The author and SOMA Wellness Clinic disclaim all liability for any outcomes resulting from the application of the information provided. Always consult a licensed physician before beginning any new therapy.